Iron: The Fundamental Nutrient for Life1,2
Iron is much more than just another mineral; it’s an essential building block of life, intricately involved in numerous physiological processes critical to human health. Found within every living cell, iron plays pivotal roles in oxygen transport, energy metabolism, cell growth, and immune system functionality. Without sufficient iron, cells cannot produce energy effectively, cognitive functions can diminish, and the body’s ability to combat infections is significantly compromised. Iron acts as a catalyst in the synthesis of neurotransmitters, impacting mood and cognitive performance, and it supports enzymes necessary for DNA synthesis and repair. Hence, maintaining adequate iron levels is not merely about avoiding anemia; it is about ensuring the optimal function and vitality of the entire body, sustaining health and promoting overall well-being.
Iron’s Essential Functions3–5
At the heart of iron’s importance is its role in oxygen transport. The majority of iron in the body resides within two essential proteins:
Hemoglobin, found in red blood cells, transports oxygen from the lungs to tissues throughout the body.
Myoglobin, present in heart and skeletal muscles, stores oxygen, releasing it during periods of high demand, such as intense physical activity.
Additionally, iron is crucial for energy production, DNA synthesis, and maintaining a robust immune system. Approximately 15–30% of the body’s iron reserves are stored as ferritin, primarily within the liver, spleen, and skeletal muscles, ready to replenish iron levels as needed.
Facts About Iron
Iron comes into the gut from the diet as Fe3+, in the stomach its converted to Fe+2 form with help of stomach acid(Hcl) and Vit-C.
Fe2+
is heme Iron Fe3+
is non-heme Iron Only Fe2+ is absorbed via Duodenum through the Heme Carrier Protien-1 and Divalent mMetal transporter-1 (DMT-1).
When Fe²⁺ is absorbed from the gut, it is oxidized to Fe³⁺ by ferroxidases (e.g., hephaestin, ceruloplasmin) before loading onto transferrin to ensures no free Fe²⁺ is circulating in plasma in order to avoid Fenton reaction.
The Fenton reaction is a chemical reaction in which ferrous iron (Fe²⁺) reacts with hydrogen peroxide (H₂O₂) to produce highly reactive hydroxyl radicals (•OH).
In iron deficiency there is:
- Decrease level of Ferritin (Stored Iron in the cell)
- Decrease level of serum iron
- Increase in the serum Total iron binding capacity (TIBC)
- Decrease in the Transferrin satuartion (Iron/TIBC x 100)
- Increase in soluble transferrin receptor concentration (sTfR)
Understanding Iron Deficiency6,7
Iron deficiency, a prevalent nutritional shortfall worldwide, occurs when iron levels dip below the body’s requirements, disrupting normal physiological processes. This deficiency can subtly manifest as cognitive impairments—such as decreased memory and difficulty concentrating—and reduced immune resilience, making the body susceptible to frequent illnesses.
Significantly, iron deficiency impacts physical performance, triggering debilitating fatigue and diminished exercise tolerance. Pregnant women facing iron deficiency are at increased risk for adverse outcomes, including preterm birth and low birth weight infants. Infants born with iron deficiency may encounter developmental delays, both physically and mentally.
What is Iron Deficiency and Iron Deficiency Anemia?8
Iron deficiency is a condition in which the body lacks sufficient iron to maintain normal physiological functions. It initially manifests subtly and progresses if unaddressed.
When iron deficiency becomes severe enough to significantly impair hemoglobin synthesis, it leads to a condition known as iron deficiency anemia.
Iron deficiency anemia is characterized by a reduced number of red blood cells or lower hemoglobin concentration within these cells, resulting in impaired oxygen transport throughout the body.
Symptoms include:
Fatigue
Pallor
Shortness of breath
Dizziness
Cognitive impairment
Early identification and treatment of iron deficiency are crucial to prevent progression to iron deficiency anemia and its associated complications.
Identifying Iron Deficiency9–11
Signs and Symptoms
General symptoms of iron deficiency include:
- Persistent fatigue
- Muscular weakness
- Shortness of breath
- Dizziness or headaches
- Coldness in extremities
- Noticeable pallor in skin tone
Other subtle indicators may include brittle nails, soreness or swelling of the tongue, chest pain, and unusual cravings—known as pica—for non-nutritive substances such as ice, dirt, paint, or starch. Daily functions such as exercise capacity, school and work performance, and libido can also be negatively affected.
Underlying Causes of Iron Deficiency12–14
Iron deficiency typically arises from three main avenues:
Blood Loss:
Heavy menstruation, internal gastrointestinal bleeding, frequent blood donations, and trauma or surgery-related blood loss.
Dietary Insufficiency:
Diets lacking iron-rich foods, common in restrictive diets like vegetarianism or veganism, can lead to deficiency, especially during pregnancy and childhood.
Reduced Absorption:
Conditions like inflammatory bowel disease, Coeliac disease, gastric bypass surgeries, and medications reducing stomach acid (e.g., proton pump inhibitors) severely impair iron absorption.
Who is Most at Risk?
Specific populations particularly susceptible to iron deficiency include:
- Pregnant women and adolescent girls
- Young children experiencing rapid growth
- Individuals adhering to restrictive diets
- Patients with chronic conditions, including kidney disease requiring dialysis, gastrointestinal disorders, and chronic intestinal infections
- Socially disadvantaged groups such as the elderly in care facilities, indigenous populations, refugees, and economically disadvantaged migrants
- Regular blood donors and elite athletes
- Individuals with chronic gastrointestinal blood loss due to prolonged NSAID use or those who’ve undergone weight-loss surgeries
Advancements in Detection and Diagnosis15–17
Modern medicine has significantly advanced the detection and diagnosis of iron deficiency, improving patient outcomes through precise and timely interventions. Traditional diagnostic measures like haemoglobin and serum ferritin remain valuable but can be limited by conditions complicated by chronic inflammation or kidney disease. Consequently, novel biomarkers are gaining attention such as:
- Hepcidin, a liver-derived hormone, provides critical insights into iron homeostasis. Hepcidin controls iron absorption from the intestine and its release from storage sites by binding to and inducing degradation of ferroportin, the cellular iron exporter. Elevated hepcidin indicates restricted iron availability and impaired absorption, frequently associated with chronic inflammatory conditions.
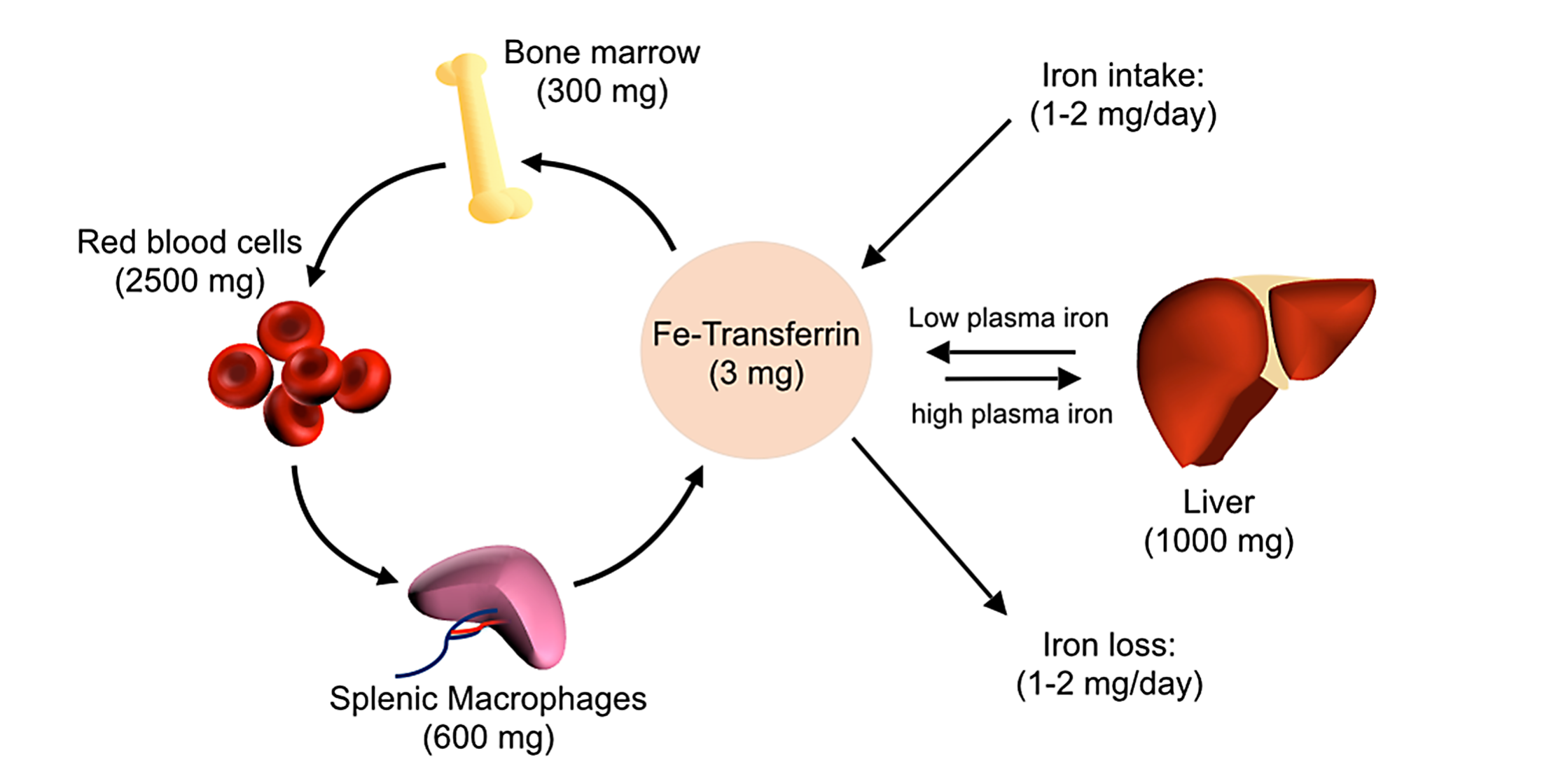
Iron homeostasis: Iron is absorbed from the diet by duodenal enterocytes and transported into the bloodstream, where it is bound by transferrin. Most iron is incorporated into erythrocytes for heme synthesis. Splenic macrophages recover iron from senescent erythrocytes and releaseiron into circulation via ferroportin. Smaller amounts of iron are imported into other tissues as needed. Iron loss is not directly regulated and occurs through minor bleeding and shedding of duodenal enterocytes.
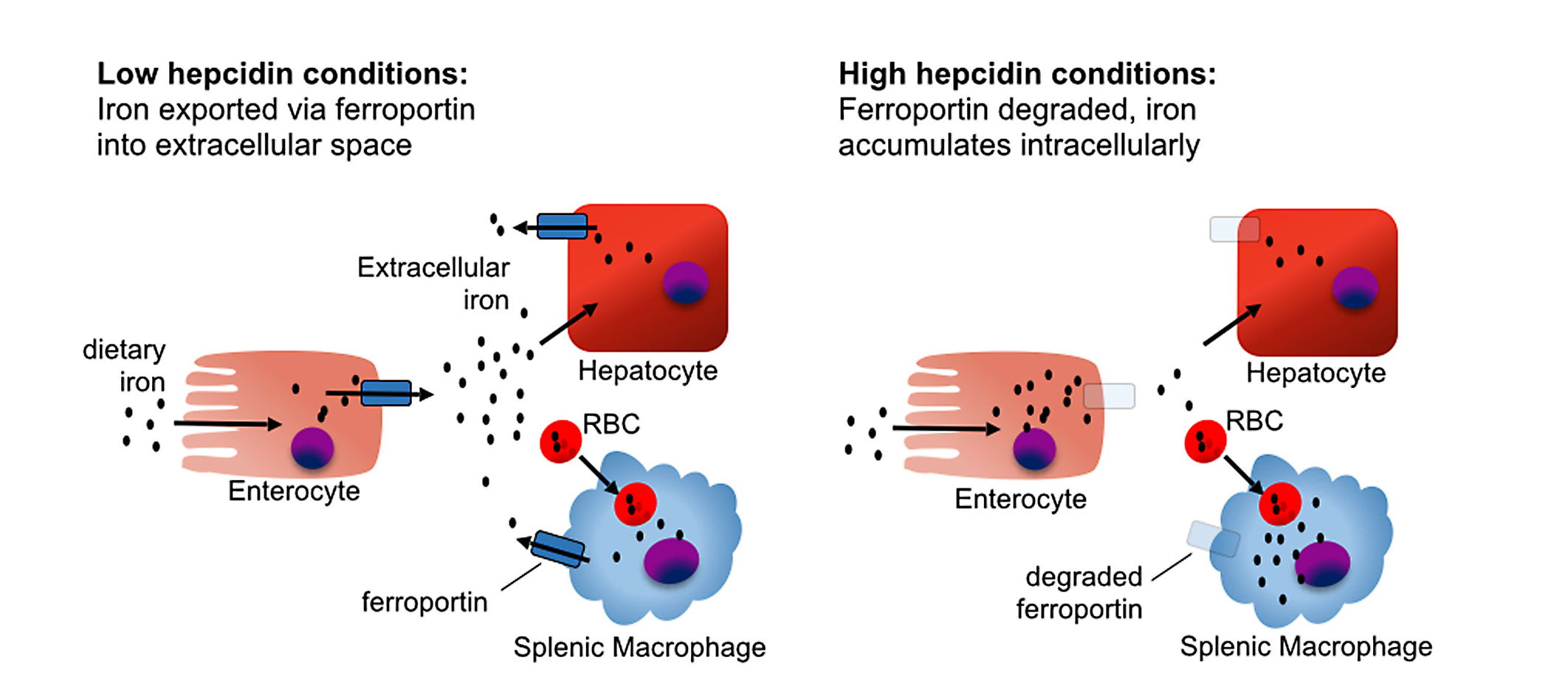
- Transferrin receptor (sTfR), is another soluble key biomarker, which elevates when cells experience iron deficiency. Unlike ferritin, sTfR levels are less influenced by inflammation, making this marker particularly beneficial for diagnosing iron deficiency in patients with inflammatory conditions or infections, where traditional markers can be misleading.
Recent diagnostic strategies involve comprehensive iron studies, integrating parameters such as:
Serum iron levels
Total iron-binding capacity (TIBC)
Transferrin saturation
Reticulocyte haemoglobin content.
Such integrative approaches offer a holistic view of an individual’s iron status, enabling more accurate therapeutic decisions.
Advancements in imaging technologies, including magnetic resonance imaging (MRI) techniques like R2 and R2* relaxometry, have allowed clinicians to non-invasively quantify tissue iron concentrations, especially in critical organs such as the liver and heart. This is particularly valuable for managing chronic iron overload in patients undergoing regular transfusions like thalassemia or sickle cell anemia.
Treatment Strategies18–21
Managing iron deficiency involves restoring iron stores and normalizing haemoglobin levels. Treatment strategies include:
Dietary Intervention:
Incorporate iron-rich foods (red meat, poultry, fish, iron-fortified cereals, pulses, dried fruits, spinach) and vitamin C-rich foods (citrus fruits, broccoli, tomatoes) to enhance iron absorption. Avoid foods that inhibit iron absorption, like tea, coffee, high fibre foods, alcohol, and dairy products.
Iron Supplementation:
- Multivitamin-mineral supplements like Spatone®, Floradix®, Mega Iron®, FAB Iron® (AUST-L products).
In Australia, AUST-R and AUST-L are codes used by the Therapeutic Goods Administration (TGA) to show how a medicine is regulated:
AUST R (Registered Medicines) Includes prescription medicines, most over-the-counter medicines, and some higher-risk complementary medicines.
Assessed by the TGA for:
Quality
Safety
Efficacy
AUST L(Listed Medicines) Lower-risk medicines, such as many vitamins, minerals, herbal supplements.
Assessed by the TGA for:
Quality
Safety
Not assessed for efficacy
The sponsor is responsible for ensuring claims are correct, but the TGA does not evaluate effectiveness before listing.
Many iron-containing supplements available do not have adequate elemental iron to be effective for iron deficiency.
- Therapeutic oral iron preparations like Maltofer® Syrup and Ferro-grad C® ( AUST-R products) provide sufficient elemental iron (approximately 100 mg/dose) for effective treatment.
Newer iron supplement formulations, such as iron polymaltose complexes (e.g., Maltofer®), are recognized for fewer gastrointestinal side effects compared to traditional iron salts, improving patient compliance.
Side Effects and Interactions22–24
Side Effects (affect ~4 in 10 people):
- Constipation
- Diarrhoea
- Stomach upsets
- Nausea and/or vomiting
Interactions – Factors that reduce iron absorption:
Food interactions: Taking iron with food such as wheat, dairy, tea, or coffee may reduce iron absorption.
Drug interactions: Iron absorption may be reduced by:
Antacids
H2 antagonists
Proton pump inhibitors (PPIs)
Calcium supplements
Conclusion
Iron is the fuel that keeps your body and brain moving. When levels run low, tiredness, breathlessness, and “foggy” thinking often follow. The path back is straightforward: confirm the diagnosis with ferritin and transferrin saturation (and hepcidin/sTfR if inflammation is suspected), address the cause, optimise diet and absorption, choose an appropriate iron preparation, and recheck in 4–8 weeks. With this steady, evidence-based approach, most people quickly regain their energy, focus, and performance— and maintain those gains with good adherence and follow-up.