Introduction
Few medicines are as universally trusted and widely used as paracetamol (also known as acetaminophen). Whether it’s for relieving a mild headache, bringing down a fever, or easing the discomfort of muscle pain, this everyday drug is a staple in households around the globe. Yet, behind its simplicity lies a manufacturing process deeply tied to petrochemicals—derived from non-renewable resources and often environmentally taxing. What if we could produce paracetamol from something we’re desperate to get rid of instead—plastic waste?
Welcome to the frontier of green chemistry, where scientists have managed to do exactly that by teaching E. coli, a humble bacterium, to convert plastic into this essential drug.
The Pharmacology of Paracetamol: A Quick Dive1
Lets dive first into the Quick guide to paracetamol
💊 Quick Guide: Paracetamol
Therapeutic Use
- Headache and migraine
- Toothache, sore throat
- Muscular aches, arthritis, osteoarthritis
- Period pain, rheumatic pain
- Cold & flu symptoms
- Fever reduction
Contraindications
- Hypersensitivity to paracetamol or excipients
- Liver impairment
- G6PD deficiency
Drug Interactions
- ↑ Warfarin bleeding risk
- ↑ Absorption: metoclopramide, domperidone
- ↓ Absorption: cholestyramine, anticholinergics
- ↑ Toxicity with antiepileptics, rifampicin, alcohol
- Avoid with zidovudine
Pharmacokinetics
- Absorption: Rapid, peak 10–60 mins
- Distribution: Crosses placenta, enters milk
- Metabolism:In the Liver through NAPQI pathway. It is a toxic metabolite of paracetamol (acetaminophen) produced by the cytochrome P450 enzyme system (primarily CYP2E1).Under normal circumstances, NAPQI is detoxified by conjugation with glutathione
- Excretion: Urine; half-life ~1–4 hours
⚠️ Overdose
- Toxic dose: >10–15 g may cause liver failure
- Early signs: nausea, vomiting, abdominal pain
- Late signs: jaundice, coma, hepatic failure
- Treatment: IV Acetylcysteine (N-acetylcysteine,NAC) and Oral Methionine
Dosage & Administration
Adults & Children ≥12 years:
1–2 tablets (500mg) every 4–6 hours; max 8/day
Children (7–12 years):
½ to 1 tablet every 4–6 hours; max 4/day
Child (4–6 years):
240mg every 4–6 hours (max 4 doses in 24 hours)
Child (2–4 years):
180mg every 4–6 hours (max 4 doses in 24 hours)
Child (6 month–2 years):
120mg every 4–6 hours (max 4 doses in 24 hours)
Child (3–6 months):
60mg every 4–6 hours (max 4 doses in 24 hours)
Child (2–3 months):
60mg may be repeated once after 4–6 hours if needed
Warnings & Precautions
- Hepatotoxicity risk even at normal doses
- Use caution in alcohol use, renal issues, malnutrition
- Severe skin reactions: Stevens-Johnson Syndrome(SJS), Toxic Epidermal Necrolysis(TEN)
Pregnancy & Lactation
- Pregnancy Category A: Safe if needed
- Excreted in breast milk in very low amounts
Pharmacodynamics
Mechanism of Action:
Acts centrally to inhibit COX enzymes, reducing prostaglandins linked to pain and fever. Minimal anti-inflammatory activity.
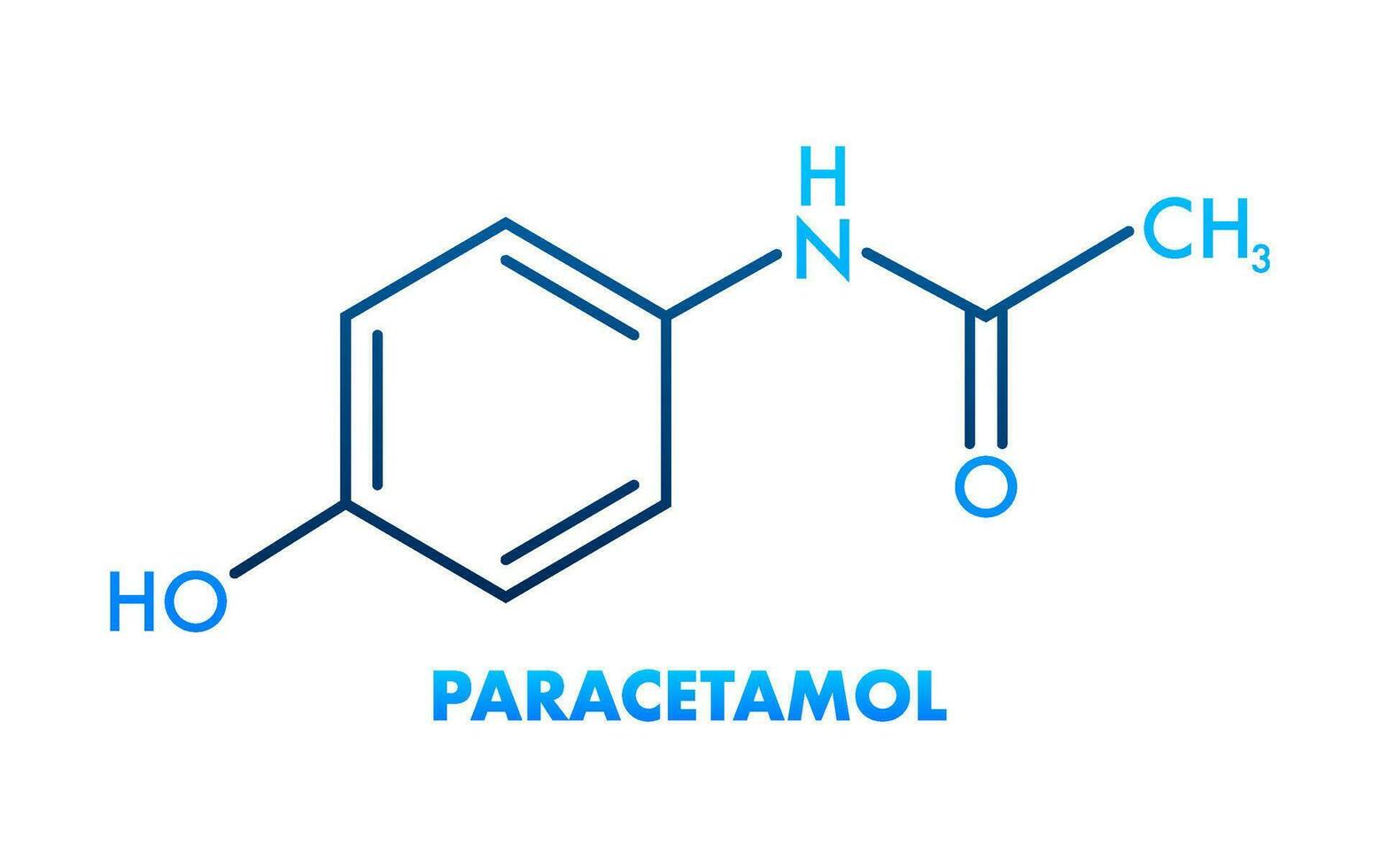
Chemistry
- Chemical name: N-acetyl-p-aminophenol
- Formula: C₈H₉NO₂
- Molecular weight: 151.17
Treatment of Overdose
In the treatment of paracetamol overdose, the antidotes used are IV acetylcysteine and oral methionine, depending on timing, availability, and clinical context. Here are the standard dosing regimens:
IV Acetylcysteine (N-acetylcysteine, NAC)
Standard 21-hour protocol (3 infusion regimen):
1st dose: 150 mg/kg over 1 hour (diluted in 200 mL of 5% glucose or sodium chloride 0.9%)
2nd dose: 50 mg/kg over 4 hours (diluted in 500 mL)
3rd dose: 100 mg/kg over 16 hours (diluted in 1000 mL)
Total dose over 21 hours: 300 mg/kg
Oral Methionine
Used when IV NAC is unavailable and if treatment can be started within 10 hours of overdose.
Dose:
2.5 g every 4 hours for 4 doses (total 10 g) Children: 1 g every 4 hours for 4 doses Methionine should be given with antiemetics if vomiting is a concern.
From Pill to Plastic: A Radical Reimagining2,3
In a groundbreaking study published in Nature Chemistry (June 2025), Johnson and his team redefined what is chemically and biologically possible. The team successfully programmed Escherichia coli (E. coli)—a common gut bacterium—to convert PET plastic waste into paracetamol through a novel blend of synthetic chemistry and metabolic engineering.
Here’s how it unfolds:
Step 1 Plastic Breaks Down
They started with PET plastic (polyethylene terephthalate), found in most plastic bottles. Using chemical methods, PET was broken down into smaller building blocks, one of which was turned into a compound called N-acyl hydroxamate.
This molecule is the starting point for a powerful transformation: the Lossen rearrangement
Step 2 The Lossen Reaction — Now Inside a Living Cell
The Lossen rearrangement is a chemical reaction, named after German chemist Wilhelm Lossen, who discovered it in 1872, where a hydroxamate converts into an isocyanate intermediate, which then becomes an amine. This reaction usually requires harsh lab conditions—high temperatures or chemical catalysts.
But here’s the twist: the researchers made it happen inside living E. coli cells, with nothing more than phosphate ions (naturally present in the cells) to drive the reaction. This is the first time a non-enzymatic rearrangement like this was shown to work in a living system.
The result? The bacteria converted the hydroxamate into PABA—short for para-aminobenzoic acid.
Step 3 Why PABA Matters
PABA is a key intermediate in folate synthesis. E. coli normally makes it on its own, but the scientists used a strain that couldn’t produce PABA unless it came from the Lossen rearrangement. This setup served as a biological proof that the reaction was indeed happening inside the cells.
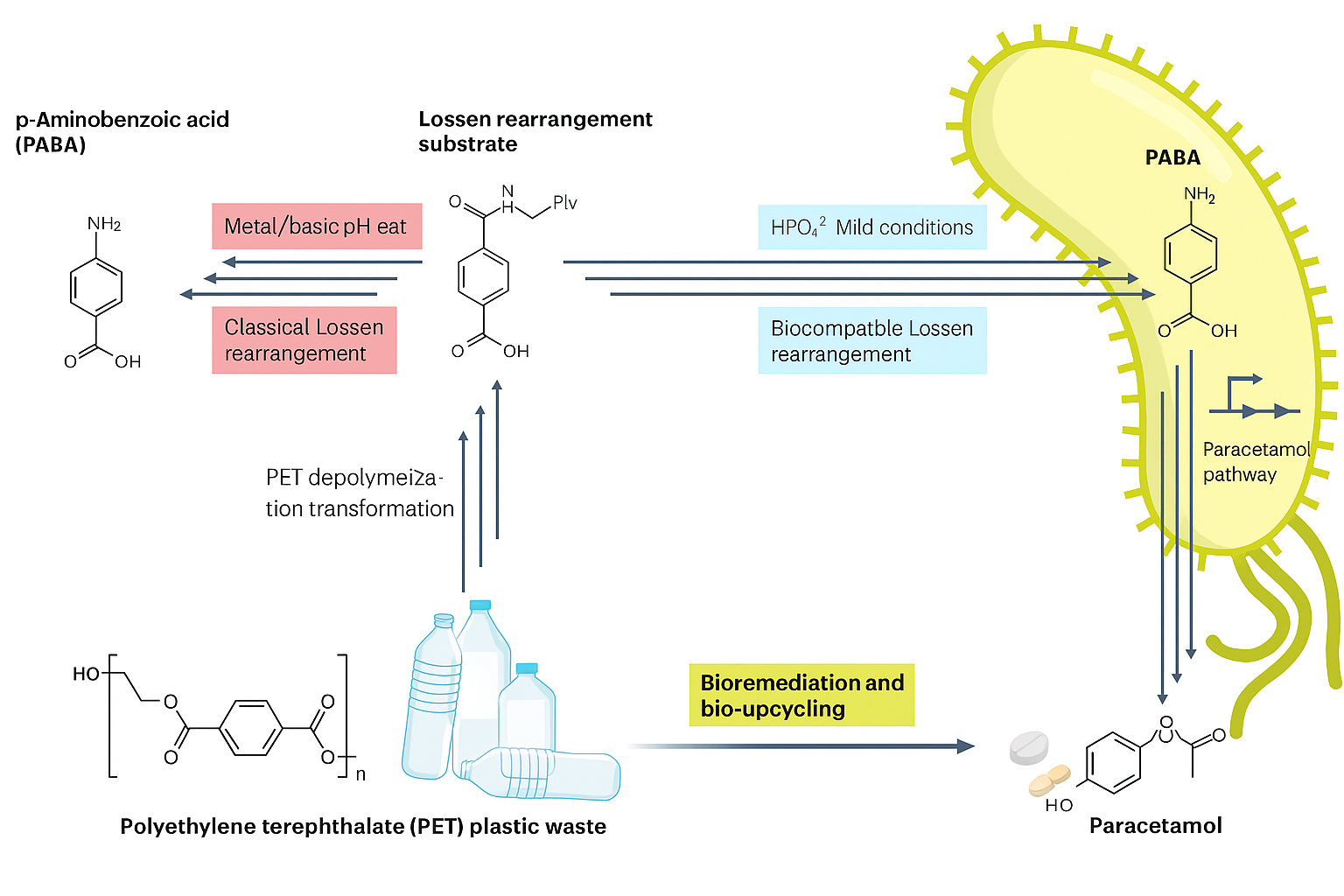
Step 4 Making Paracetamol
Next, the team engineered the bacteria to convert PABA into paracetamol. This required adding two foreign genes:
Arylamine N-acetyltransferase (nat) from a mushroom (Agaricus bisporus) – converts PABA into N-acetyl-PABA.
Amidase (amiE) from a soil bacterium (Pseudomonas putida) – completes the final step to form paracetamol.
With both genes active, the engineered E. coli could turn plastic-derived molecules into paracetamol in a single living system.
This innovation fuses science and sustainability to transform waste into life-saving medicine.
Why is This Important4,5
Green chemistry at its best
This method avoids the use of petroleum and hazardous reagents. It operates at mild temperatures, in water, using living cells.92% of the PET-derived substrate was transformed into paracetamol in 24–48 hours at ~37 °C—phenomenal efficiency for a cell-based synthetic process
A milestone in synthetic biology
Performing a Lossen rearrangement inside a living cell opens the door for more in vivo applications of traditional organic chemistry—blending synthetic chemistry and biology.
Upcycling plastic waste
Turning environmental pollutants into life-saving medicines not only offers a novel recycling route but reframes how we view waste as a raw material.
Conclusion
The transformation of plastic waste into a life-saving medication like paracetamol is more than a technical achievement—it’s a powerful symbol of what science can accomplish at the intersection of chemistry, biology, and sustainability. By harnessing the synthetic potential of engineered microbes, scientists have not only challenged the traditional boundaries of organic chemistry but also offered a radical new vision for pharmaceutical manufacturing—one that is clean, circular, and resource-efficient.
The path from plastic to paracetamol is not just a clever detour; it may very well be the road ahead