Overview1–4
Obesity has evolved from a personal health issue to a global crisis of epidemic proportions. Since 1975, obesity rates have nearly tripled worldwide, driven by changes in lifestyle, diet, and socioeconomic structures. Today, more than 650 million adults and 340 million children and adolescents live with obesity. In the United States alone, over two-thirds of adults are overweight or obese, while in the United Kingdom, nearly one in four adults and one in five children aged 10–11 are affected.
Obesity is classified using the body mass index (BMI), where a BMI ≥30 kg/m² indicates obesity for most populations. However, for ethnic groups such as South Asians and African-Caribbeans, lower BMI cutoffs are used due to heightened risk at lower fat thresholds. Though once stigmatized as a consequence of poor self-control, obesity is now increasingly recognized as a complex, relapsing, and chronic disease with profound implications for public health and healthcare systems.
The burden is not just personal—obesity raises the risk of type 2 diabetes, cardiovascular disease (CVD), numerous cancers, respiratory dysfunction, and mental illness. Economically, the toll is staggering: over $190 billion is spent annually in the U.S. alone on managing obesity-related conditions.
Most prominent metabolic and psychological comorbidities associated with morbid obesity are elegantly illustrated in the imagee.
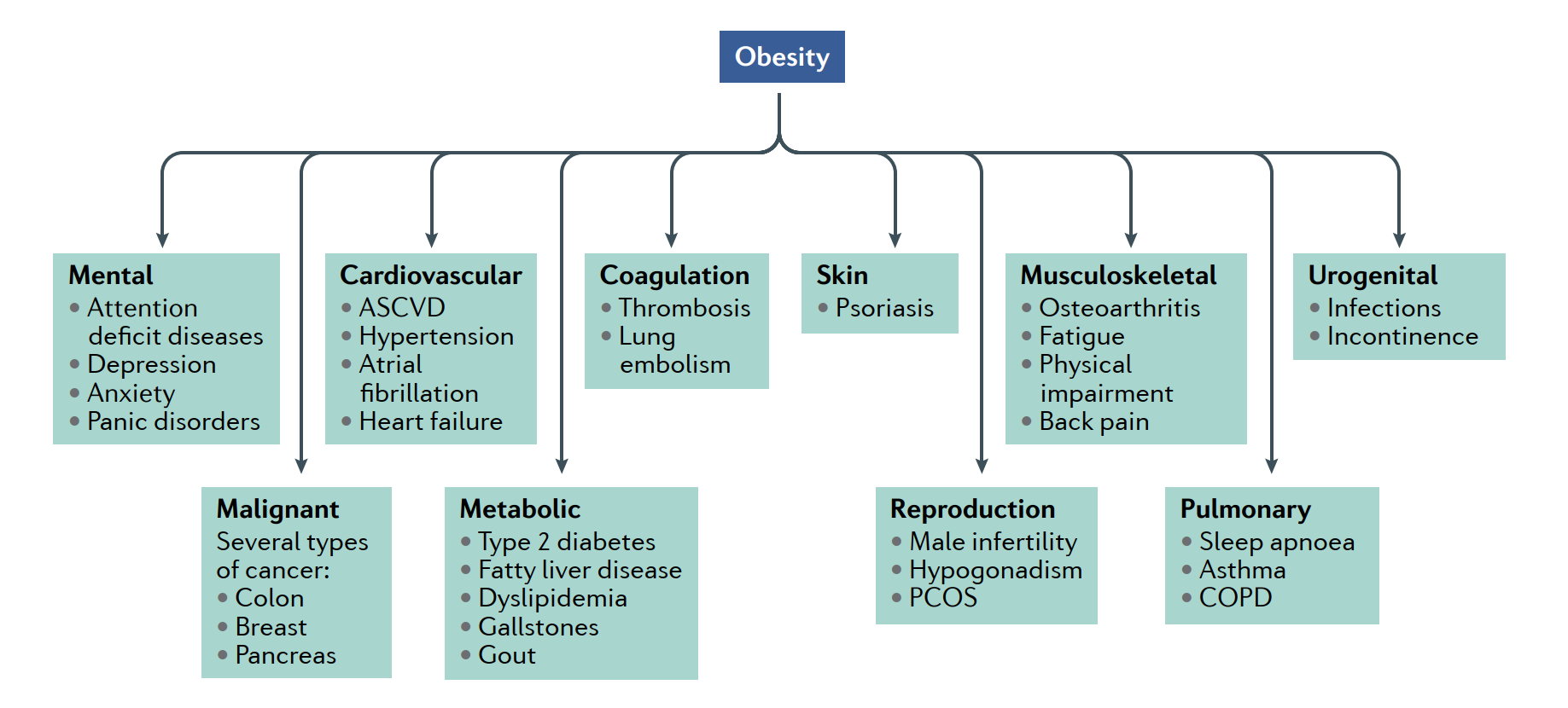
ASVCD: Atherosclerotic cardiovascular disease; COPD: Chronic obstructive pulmonary disease; PCOS: Polycystic ovary syndrome.
Causes5–7
Lifestyle and Environmental Triggers
Obesity emerges from chronic energy imbalance—caloric intake consistently exceeding expenditure. High-calorie processed foods, sedentary occupations, and mechanized transport contribute significantly to the modern obesogenic environment.
Circadian Disruption, Sleep, and Stress
Sleep deprivation, circadian misalignment, and chronic psychological stress are increasingly linked to weight gain, as they disrupt hormonal regulation of appetite and satiety.
Medications and Endocrine Disorders
Weight gain may be triggered by medications such as corticosteroids, psychotropics, and antiepileptics, or rare medical conditions like hypothyroidism and Cushing’s syndrome.
Genetics and Epigenetics
With heritability estimates between 40–70%, genetics plays a significant role. Mutations in the MC4R and FTO genes are associated with obesity risk, and rare monogenic conditions such as leptin or POMC deficiency underscore the biological underpinnings.
What is POMC?
POMC stands for Pro-opiomelanocortin, a precursor polypeptide that gets cleaved into multiple biologically active peptides, including:
- ACTH (Adrenocorticotropic hormone) – stimulates cortisol release from adrenal glands.
- α-MSH (Alpha-melanocyte stimulating hormone) – reduces appetite and increases energy expenditure by acting on MC4 receptors in the hypothalamus.
- β-Endorphin – involved in pain modulation and reward.
Role in Appetite and Obesity
In the hypothalamus, particularly in the arcuate nucleus, POMC neurons release α-MSH, which binds to melanocortin-4 receptors (MC4R) to suppress appetite and stimulate energy expenditure.
POMC deficiency, whether genetic or functional, results in:
- Uncontrolled appetite (hyperphagia)
- Early-onset severe obesity
- Often red hair and pale skin (due to lack of MSH effects on melanocytes)
- Adrenal insufficiency (due to lack of ACTH)
Diagnosis8,9
BMI remains the primary tool for screening, but its limitations have prompted supplementary measures. Although simple and widely accessible, BMI does not differentiate between fat and lean mass, nor does it provide insight into fat distribution or associated health risks.
Consequently, additional metrics—such as waist circumference and waist-to-height ratio—are now recommended to better assess cardio-metabolic risk. A waist-to-height ratio of ≥0.5 or a waist circumference >94 cm in men and >80 cm in women is considered a stronger predictor of obesity-related complications, particularly when abdominal fat is predominant.
Ethnicity further complicates interpretation, as populations of South Asian, African, Middle Eastern, and some Indigenous backgrounds often experience metabolic complications at lower BMI thresholds. This has led to the adoption of population-specific cut-offs to more accurately reflect disease risk.
Modern clinical assessment now takes a broader view, incorporating not only anthropometry but also blood pressure, fasting glucose, lipid profiles, and evaluation of lifestyle, dietary behaviors, and mental health status. Leading experts and international commissions have called for a shift toward complication-centric obesity diagnosis—one that recognizes the presence and severity of metabolic dysfunction, rather than relying solely on body size
Management
Lifestyle Intervention10–12
The cornerstone of management remains a calorie-controlled diet and physical activity:
Diet
Reducing intake by 500–600 kcal/day can yield weight loss of 0.5–1 kg per week. A diet rich in fiber, vegetables, whole grains, and lean protein is ideal.
Exercise
Adults should aim for 150–300 minutes of moderate-intensity exercise weekly, or up to 60–90 minutes daily to prevent weight regain.
Behavioral Support
Counseling and cognitive behavioral therapy (CBT) can improve adherence and address psychological drivers of obesity.
lifestyle changes alone are insufficient due to powerful homeostatic mechanisms.
However, for many individuals, lifestyle changes alone are insufficient due to powerful homeostatic mechanisms that defend body weight—even after modest weight loss.
For instance, when you lose weight, your body may respond by:
Reducing metabolism (burning fewer calories)
Increasing hunger (making you want to eat more)
Triggering hormonal changes that make it easier to regain the lost weight
As a result, sustaining weight reduction becomes increasingly difficult over time, even for the most motivated individuals. This challenge has fueled a global race among researchers and pharmaceutical companies to develop effective anti-obesity drugs—medications designed to target the body’s weight-regulating systems and support long-term metabolic health
The Race of Anti-Obesity Drugs13,14
Pharmaceutical innovation in obesity treatment has historically trailed behind advancements in fields like diabetes and cardiovascular care. For decades, obesity was viewed primarily through a lifestyle lens, and pharmacologic interventions were few—and often flawed. But in recent years, the landscape has transformed. We are now witnessing a renaissance: a competitive and scientifically exciting race among biotech companies and academic institutions to develop the next generation of anti-obesity medications (AOMs).
Past Failures and Lessons Learned
The history of AOMs is marked by cautionary tales. Early agents such as Sibutramine and Rimonabant were withdrawn due to adverse cardiovascular and psychiatric effects. Even Orlistat, still approved and available over the counter, provides only modest weight loss—often limited by gastrointestinal side effects. These setbacks contributed to decades of inertia, with few new options reaching patients. However, they also laid the groundwork for a more cautious and mechanistically informed approach.
📋 Click to View: History of Weight Loss Drugs
| Class | Drug | Side effects | Approval |
| Mitochondrial uncoupler | DNP | Hyperthermia, tachycardia, fever, tachypnoea, death | Stanford University 1933–1938 (USA) |
| Sympathicomimetic | Diethylpropion/afepramone | Nausea, constipation, insomnia, headache, tension and irritation, seizures | Merrell National Drug 1959–present (EU) |
| Sympathicomimetic | Methamphetamine | High risk for abusiveness and addiction | Abbott Laboratories 1947–1979 (USA) |
| Sympathicomimetic | Phenmetrazine | Nausea, diarrhoea, dry mouth | Ciba-Geigy Corp 1956–present (USA) |
| Sympathicomimetic | Phendimetrazine | Nausea, diarrhoea, dry mouth | Carnick Laboratories 1959–present (USA) |
| Sympathicomimetic | Phenylpropanolamine | Haemorrhagic stroke | Thompson Medical 1960–2000 (USA) |
| Sympathicomimetic | Fenfluramine and dexfenfluramine | Cardiac valvular insufficiency and pulmonary hypertension | Wyeth Ayerst 1973–1997 (USA) |
| Sympathicomimetic | Cathine (nor-pseudoephedrine) | Tachycardia, increased BP, restlessness, insomnia, depression | Riemser Pharma 1975–present (EU; short-term only) |
| Sympathicomimetic | Sibutramine | Non-fatal myocardial infarction and stroke (in individuals with pre-existing CVD) | Abbott Laboratories 1997–2010 (USA/EU) |
| Sympathicomimetic | Phentermine | Palpitations, elevated blood pressure | Teva Pharmaceuticals 1959–present (USA/EU; short-term only) |
| Polypharmacy | Rainbow pills | Insomnia, palpitations, anxiety, ↑ heart rate and BP, death | Clark & Clark and others 1961–1968 (USA) |
| CB1 receptor blocker | Rimonabant | Depression, suicidal ideation | Sanofi SA 2006–2009 (EU) |
| Pancreatic lipase inhibitor | Orlistat | Liver injury, gastrointestinal symptoms | Roche Pharmaceuticals 1999–present (USA/EU) |
| 5-HT2c serotonin agonist | Lorcaserin | Depression, suicidal ideation, palpitations, GI symptoms, ↑ cancer risk | Arena Pharmaceuticals, Eisai 2012–2020 (USA) |
| Sympathomimetic/anticonvulsant | Phentermine/topiramate ER | Depression, suicidal ideation, cardiovascular events, memory loss, birth defects | Vivus 2012–present (USA) |
| Opioid receptor antagonist/dopamine and noradrenaline reuptake inhibitor | Naltrexone SR/bupropion SR | Seizures, palpitations, transient blood pressure elevations | Orexigen Therapeutics Inc. 2014–present (USA/EU) |
| GLP1R agonists | Liraglutide | Nausea/vomiting, diarrhoea, constipation, pancreatitis, gallstones | Novo Nordisk 2014–present (USA/EU) |
| GLP1R agonists | Semaglutide | Nausea/vomiting, diarrhoea, constipation | Novo Nordisk 2021–present (USA) |
The GLP-1 Breakthrough
A true turning point came with the rise of GLP-1 receptor agonists, initially developed for type 2 diabetes. These agents, which mimic the gut hormone glucagon-like peptide-1, enhance satiety, delay gastric emptying, and influence reward-related neural pathways—resulting in significant weight loss.
Liraglutide (3.0 mg daily) demonstrated up to 8% reduction in body weight over one year.
Semaglutide (2.4 mg weekly), in contrast, delivered transformative results—up to 15% body weight loss, nearing the efficacy of bariatric surgery.
Bariatric Surgery
Weight loss surgery, also called bariatric surgery,is sometimes used to treat people with severe obesity who fulfil all of the following criteria:
- They have a BMI of 40 or more.
- They have a serious health condition that could be improved with weight loss, such as type 2 diabetes or high blood pressure.
- The person is fit enough to have anaesthesia,surgery and commits to the need for long-term follow-up
These therapies redefined expectations for medical weight loss and ushered in a new pharmacological era.
Tirzepatide: A Game-Changer
Even more potent is tirzepatide, a dual GIP/GLP-1 receptor agonist. By combining the effects of two incretin hormones, it has outperformed semaglutide in head-to-head trials, achieving up to 20% weight loss and robust glycaemic improvements. This dual agonist represents a new gold standard in AOM efficacy, proving that rationally engineered peptides can produce results once thought possible only through surgery
Multi-Agonists and the Future Pipeline
As the science evolves, the next frontier lies in multi-agonist therapies such as GLP-1/GIP/glucagon tri-agonists—and molecular mimics of bariatric surgery effects. These aim to address multiple physiological pathways simultaneously, maximizing metabolic benefits while minimizing side effects.
Molecular Mimics of Bariatric Surgery
Bariatric(weight loss) surgeries like gastric bypass (RYGB) or sleeve gastrectomy are highly effective against obesity and type 2 diabetes due to their metabolic effects, not just mechanical restriction of food.
Scientists are now creating drugs that reproduce the same hormonal changes induced by surgery, such as:
- Increased GLP-1 and PYY (appetite-suppressing gut hormones)
- Reduced ghrelin (hunger hormone)
- Improved insulin sensitivity
- Altered bile acid signaling and gut microbiota
🧬 These are called Molecular Mimics because they try to imitate the same physiological cascade as surgery—without actual surgical intervention
Leptin sensitizers like celastrol and withaferin A, which help restore the brain’s responsiveness to satiety signals—are being evaluated for their potential to overcome central leptin resistance in obesity.
Other agents moving through the development pipeline include:
Setmelanotide: Designed for individuals with rare monogenic obesity syndromes, such as POMC or LEPR deficiency.
Amylin analogues: Co-administered with GLP-1 agents to enhance appetite suppression through,distinct but complementary pathways.
Mitochondrial uncouplers: A novel class designed to boost energy expenditure without increasing caloric intake—reversing the energy imbalance at the heart of obesity
Click to View: Weight Loss Drugs in Clinical Phase
| Drug Class | Agent | Company | Phase | Clinical Trial ID |
|---|---|---|---|---|
| GLP1/glucagon dual agonists | BI 456906 | Boehringer Ingelheim | Phase II | NCT04153929 |
| GIP/GLP1 dual agonists | Tirzepatide | Eli Lilly | Phase III | NCT04657003 |
| GLP1R agonists | Rybelsus | Novo Nordisk | Phase III | NCT03919929 |
| GLP1R agonists | Danuglipron (PF-06882961) | Pfizer | Phase II | NCT04707313, NCT03985293 |
| Leptin sensitizers | Withaferin A | Academic, non-commercial | Phase I | 293 |
| Y2R agonists | NN9748 (NN9747) | Novo Nordisk | Phase I | NCT03574584 |
| Y2R agonists | NNC0165-1875 + semaglutide | Novo Nordisk | Phase II | NCT04969939 |
| Amylin analogues | Cagrilintide | Novo Nordisk | Phase II | NCT04940078, NCT04982575 |
| Other appetite suppressants | GDF15 (LA-GFD15) | Novo Nordisk | Phase I | See Related links |
| Other appetite suppressants | LY-3463251 (GDF15 agonist) | Lilly | Phase I | NCT03764774 |
Abbreviations:
GDF15, growth differentiation factor 15; GIP, glucose-dependent insulinotropic polypeptide; GLP1, glucagon-like peptide 1; GLP1R, GLP1 receptor; PYY, peptide tyrosine tyrosine; Y2R, neuropeptide Y receptor type 2; NA, not applicable.
The Rising Role of Repurposed Drugs
While novel drug design dominates headlines, drug repurposing—the strategic reapplication of existing medications—offers a powerful parallel approach. This strategy capitalizes on known safety profiles, lower development costs, and quicker clinical translation.
Among the most promising examples is Disulfiram, a long-standing treatment for alcohol dependence.
Preclinical studies have shown that disulfiram inhibits Aldehyde dehydrogenase 1 family, member A1 (ALDH1A1)*, an enzyme highly expressed in obese adipose tissue. In rodent models, it led to significant fat loss—even without reduced food intake—by enhancing metabolic efficiency in fat storage tissues.
Another candidate is Fenoprofen, an NSAID that appears to modulate neuroreceptors involved in appetite regulation, offering potential central effects on energy balance.
Meanwhile, computational screens have identified Sunitinib, a cancer therapy, for its unexpected influence on adipogenesis—revealing how large-scale bioinformatics can uncover unanticipated opportunities for weight regulation.
These repurposed agents illustrate a key advantage:
Targeting obesity’s biological drivers with already-approved drugs, accelerating their path to the clinic.
These candidates broaden the therapeutic arsenal beyond peptide hormones whether through metabolic, neuroendocrine, or inflammatory pathways.
Safety and the Road Ahead
As promising as these innovations are, safety remains the critical bottleneck. Cardiovascular outcomes, tolerability, and long-term metabolic effects will determine which therapies cross the regulatory finish line. The future likely lies in combinatorial approaches—blending the best of new peptide-based treatments, small molecules, and behavioral support for durable and safe weight control.
Conclusion
The obesity epidemic demands a multifaceted response. While diet and exercise remain foundational, the physiological defense of fat mass often undermines willpower alone. The rise of safe and effective anti-obesity drugs offers new hope—and new competition—as biotech companies vie to lead the next pharmaceutical frontier.
The current race is not just about weight loss, but about improving lifespan, metabolic health, and quality of life for millions. With semaglutide and tirzepatide as front-runners, and tri-agonists on the horizon, we may finally be entering an era where pharmacotherapy closes the gap between obesity and chronic disease prevention.