Insomnia
When you can’t sleep, the night can feel endless and the next day even longer, this is insomnia. Insomnia is one of the most common sleep disorders worldwide, and is clinically defined according to diagnostic criteria as difficulty in falling asleep, maintaining sleep or waking up too early despite adequate opportunity to sleep, leading to next day functional impairments.
To find out more about insomnia and sleep-wake cycle, see my earlier Blog article: Harnessing Nature for Restful Sleep: The Role of Herbal Extracts in Pharmaceutical and Nutraceutical Formulations
How sleep works
Your brain runs two big “Sleep Engines”:1
Carcadian Clock (SCN): Keeps time and nudging you to feel sleepy at night and alert in the morning.
SCN (suprachiasmatic nucleus): is the brain’s master body clock, tiny pair of nuclei in the anterior hypothalamus, just above the optic chiasm.
Sleep pressure (Homeostasis/Adenosine): The longer you’re awake, the stronger the drive to sleep.
Inside the brain, two hubs coordinate the dance between your body clock and sleep pressure: the wake network (RAS/orexin) and the sleep switch (VLPO).
Wake network: A relay stretching from the brainstem up through the thalamus and hypothalamus (“reticular activating system”) keeps you alert using chemical messengers like acetylcholine, dopamine, histamine, serotonin, and noradrenaline. Neurons that release orexin (a.k.a hypocretin) help stabilize wakefulness—like a seatbelt that prevents drowsy “drift.”2,3
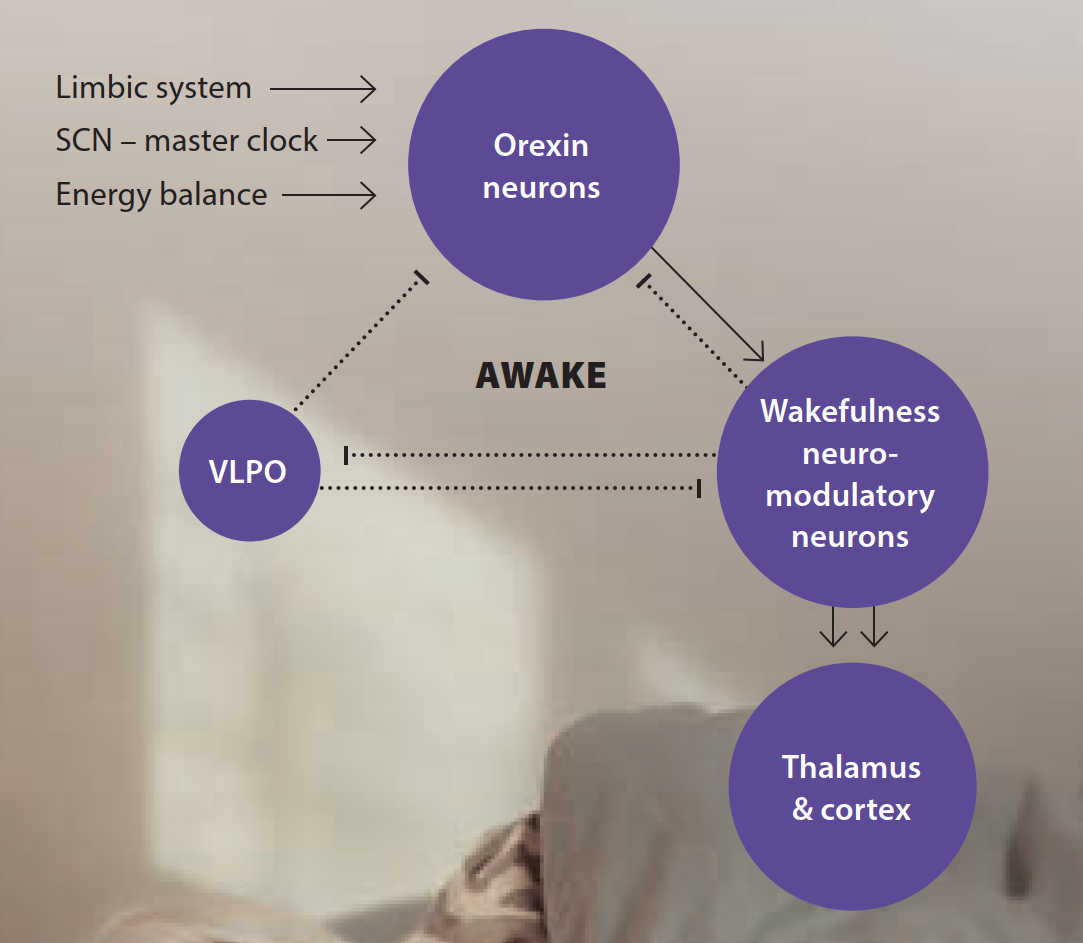
Sleep switch: In the hypothalamus, the ventrolateral preoptic area (VLPO) sends calming signals (GABA and galanin) that quiet the wake network so sleep can begin. This “mutual inhibition” forms a biological flip-flop switch—you’re either mostly awake or mostly asleep, with quick transitions.4,5
NEUROANATOMY OF THE FLIP-FLOP MODEL
SLEEP SIDE
VLPO – Ventrolateral preoptic nucleus: Located at the anterior hypothalamus, just in front/above the optic chiasm (preoptic area) and it sends GABA + galanin- strong inhibitory output.
Main targets it turns OFF:
- TMN (tuberomammillary nucleus, histamine) in the posterior hypothalamus
- LC (locus coeruleus, noradrenaline) in the dorsal pons
- DR (dorsal raphe, serotonin) in the midbrain/rostral pons
- PPN/LDT (pedunculopontine & laterodorsal tegmental nuclei, acetylcholine) at the pontine–mesencephalic junction
- Basal forebrain (nucleus basalis/medial septum—cholinergic & GABAergic) in the frontobasal cerebrum
- Also inhibits orexin neurons in the lateral hypothalamus
The MnPO—median preoptic nucleus, right next to VLPO—often works as a helper on the sleep side.
WAKE SIDE:
Arousal/neuromodulatory nuclei projecting widely to thalamus & cortex. Thes are:
- TMN
- LC
- DR
- PPN/LDT
- Basal forebrain
Plus contributors like VTA dopamine and parabrachial nucleus in the pons.
These wake centers inhibit the VLPO (GABAergic return fire), forming the mutual inhibition that makes the switch “flip-flop.”
The Stabilizer
Orexin (hypocretin) neurons: located at the lateral/perifornical hypothalamus (spilling into dorsomedial LH). and excite TMN, LC, DR, PPN/LDT, basal forebrain, parabrachial, and thalamocortical circuits → stabilise wake; also help keep the switch from slipping.
Inputs they read:
- SCN timing via the dorsomedial hypothalamus (DMH)
- Limbic signals (emotion/stress)
- Energy balance (glucose, leptin/ghrelin).
The timer
SCN (suprachiasmatic nucleus): Its role is to set when wake drive or sleep drive should peak. It routes signals (often through DMH) to orexin and VLPO, and regulates melatonin via the pineal pathway.
Final common targets
Thalamus & cortex:
When arousal nuclei (helped by orexin) are ON → activated thalamocortical networks → wake EEG, attention, responsiveness.
When VLPO wins → these pathways quieten → sleep onset and stable NREM.
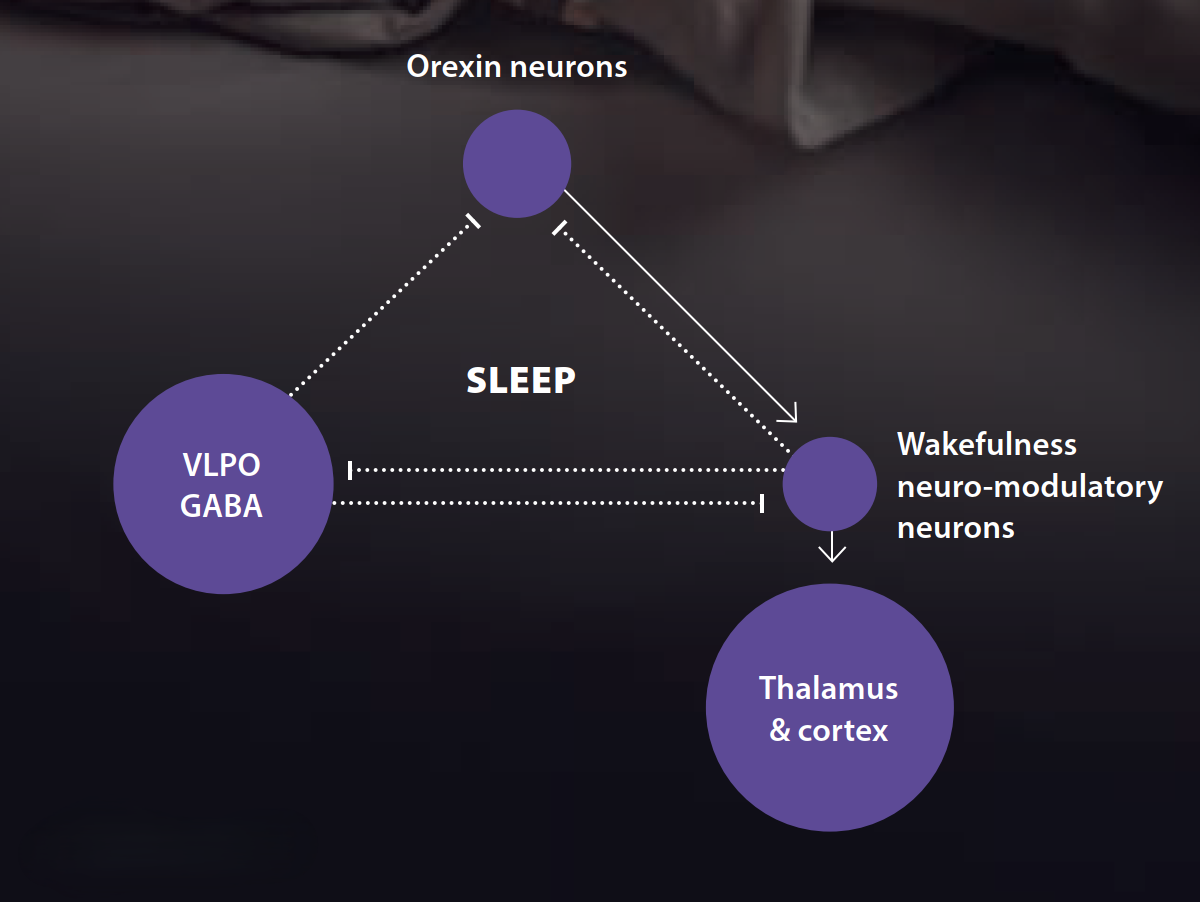
Dim light and melatonin help the VLPO; caffeine and bright light push the other way.6–9
On top of those centralized controls, “local sleep” can occur: small patches of the cortex briefly slip into sleep-like activity during prolonged wakefulness. You’re still globally awake, but these tiny “brownouts” can cause momentary lapses—typos, missed turns, clumsiness—especially when you’re up late or sleep-deprived.10
What works best right now
First-line: Cognitive Behavioral Therapy for Insomnia (CBT-I)
The strongest evidence says start with CBT-I. It’s a structured, short program (typically 4–8 weeks) that resets sleep timing and rewires unhelpful “sleep thoughts.” Key tools include sleep restriction, stimulus control, gentle relaxation, and targeted cognitive strategies. Digital programs can help when in-person therapy is hard to access.11–13
Sleep hygiene supports CBT-I, but on its own usually isn’t powerful enough for chronic insomnia.
Medicines: where they fit
Medication can be useful short-term or when CBT-I alone isn’t enough. Choosing safely matters:
Orexin receptor antagonists (DORAs):14–17 Suvorexant , lemborexant , and daridorexant block orexin’s “stay awake” signal. They tend to preserve more natural sleep stages and may cause less next-day grogginess than older sedatives for many people.
Key clinical information on dual orexin receptor antagonists
| Parameter | Lemborexant (Dayvigo) | Suvorexant (Belsomra) |
|---|---|---|
| Receptor targets | Mainly OX2; OX1 to a lesser extent | OX1 and OX2 (dual) |
| Clinical indication | Sleep-onset and sleep-maintenance insomnia | Sleep-onset and sleep-maintenance insomnia |
| Onset of action (min) | ≈ 30 | ≈ 30 |
| Volume of distribution (L) | 1,970 | 49 |
| Metabolism enzymes | CYP3A / CYP2B6 | CYP3A / CYP2C19 |
| Active metabolites | Yes — M4 / M9 / M10 | No |
| Half-life t1/2 (h) | 17–19 | 12 |
| Duration of action (h) | ~7 | ~7 |
| With/without food | Best on an empty stomach | Best on an empty stomach |
| Recommended treatment duration | Tested up to 12 months; use the shortest duration required | Reassess need for continuation after ~3 months |
| Pregnancy & breastfeeding | No data | No data |
| Hepatic/renal impairment | Avoid in severe hepatic impairment (consider lower dose in moderate) | Avoid in severe hepatic impairment |
| Key counselling point | Ensure at least 7 hours available for sleep after taking | Ensure at least 7 hours available for sleep after taking |
Avoid in narcolepsy. Don’t mix with alcohol or other sedatives.
Low-dose doxepin (3–6 mg): an older antidepressant for sleep-maintenance insomnia, repurposed at tiny doses as a selective H1 antihistamine at bedtime.18,19
Melatonin & melatonin-like drugs:20,21 Melatonin has modest effects in adults with chronic insomnia (more helpful in older adults or circadian issues like jet lag). Ramelteon (prescription melatonin-receptor agonist) shows small-to-moderate benefit with good tolerability.
Benzodiazepines and “Z-drugs” (zolpidem, zopiclone): can shorten time to fall asleep but carry risks—tolerance, dependence, memory problems, impaired coordination, falls (especially in older adults). If used, keep duration brief and review regularly.22–25
Sedating antihistamines (e.g., diphenhydramine): often not recommended for chronic insomnia due to tolerance and anticholinergic side effects.26,27
What’s new and emerging
Exciting research targets the brain’s actual sleep-wake circuitry:
Next-gen orexin blockers28–30 DORAs (above) are now established options in many countries.Selective orexin-2 antagonists (SORAs) like seltorexant focuses on the OX2 receptor (more sleep-specific). Late-stage trials in people with depression and insomnia have reported positive results, and broader insomnia trials are ongoing.
Refined GABA-A “calmers”:31–33 Dimdazenil (a partial GABA-A modulator) aims to reduce next-day impairment seen with classic benzodiazepines while still easing sleep onset; early trials show promise. EVT-201 and lorediplon are related investigational compounds.
Neuroactive steroids:34 Zuranolone (approved for postpartum depression) enhances GABA signaling and, in studies, improved insomnia symptoms alongside mood—prompting interest in targeted sleep indications. (Not a standard insomnia prescription yet.)
NOP-receptor agonists:35,36 Sunobinop (activates the nociceptin/orphanin FQ receptor) appears to boost non-REM sleep without strong next-day effects in early human studies.
A simple plan to try
- Start with CBT-I (therapist-led or high-quality digital).
- Support the basics: regular wake time, wind-down routine, dim lights 90 minutes before bed, caffeine cut-off 8+ hours before bed, alcohol avoidance.
- Consider short-term medication if insomnia is severe or CBT-I access is limited—review every few weeks. Options often considered first today include DORAs or low-dose doxepin, matched to your sleep problem (sleep-onset vs. sleep-maintenance) and your health profile.
- Use extra caution in older adults—avoid benzodiazepines and Z-drugs when possible due to fall and cognitive risks.
- If snoring, gasping, or very sleepy by day, ask about sleep apnea—treating it can transform sleep quality.
Conclusion
The most reliable long-term solution for chronic insomnia remains Cognitive Behavioral Therapy for Insomnia (CBT-I), which retrains timing, habits, and thoughts so sleep becomes more natural and stable. When medication is appropriate, today’s first-line choices increasingly favor orexin-receptor blockers and very-low-dose doxepin over older sedatives, aiming for restorative sleep with fewer next-day trade-offs. And the horizon is bright: selective orexin-2 antagonists (SORAs), refined GABA-A modulators, neuroactive steroids, and nociceptin/orphanin FQ (NOP) receptor agonists are ushering in personalized, mechanism-guided care that aligns with how the brain actually sleeps. Taken together—with a clinician’s guidance—these tools offer a modern, hopeful path back to nights that are restful and days that feel like yours again.