Introduction1
HIV (Human Immunodeficiency Virus) is a chronic retroviral infection that targets and depletes CD4+ T cells, progressively impairing immune function and leading to AIDS if untreated. Transmitted via blood, sexual fluids, and perinatal routes, HIV establishes lifelong infection by integrating into the host genome and forming latent reservoirs. While antiretroviral therapy (ART) has revolutionized disease management—enabling durable viral suppression and prolonged survival—barriers such as adherence challenges, drug resistance, stigma, and the absence of a definitive cure remain.
The therapeutic landscape has now entered a transformative phase with the advent of Lenacapavir, a first-in-class HIV-1 capsid inhibitor designed for long-acting subcutaneous delivery. Initially approved by the U.S. Food and Drug Administration (FDA) in December 2022 for treatment-experienced adults with multidrug-resistant HIV-1, Lenacapavir has since expanded its role. On June 18, 2025, the FDA approved Lenacapavir—marketed as Yeztugo—for twice-yearly pre-exposure prophylaxis (PrEP) in adults and adolescents weighing ≥35 kg, marking a pivotal advancement in both therapeutic and preventive HIV strategies
HIV Overview
Click to Expand HIV Overview Table
| Aspect | Details |
|---|---|
| Signs and Symptoms | Fever, sore throat, swollen lymph nodes, rash, muscle ache, night sweat, mouth ulcers, chills, and fatigue |
| Diagnosis | Antibody test, antigen/antibody test, and nucleic acid test |
| Prevention | Protected sex, avoiding sharing needles, HIV testing, pre-exposure prophylaxis (PrEP), and voluntary medical male circumcision |
| Challenges | Immune evasion, weakened immune system, rapid viral replication, high genetic variability, mutation, latent reservoirs, and lack of a validated animal model |
| Transmission | Unprotected sex, needle sharing, mother-to-child transmission during pregnancy or breastfeeding, contaminated fluids (blood, semen, breast milk, vaginal fluids) |
| Treatments | NRTIs (Zidovudine), NNRTIs (Nevirapine), protease inhibitors (Saquinavir), fusion inhibitors (Enfuvirtide), CCR5 antagonists (Maraviroc), integrase inhibitors (Raltegravir), and attachment inhibitors (Fostemsavir) |
Mechanism of Action: Targeting the HIV Capsid2
Distinct from conventional antiretrovirals that inhibit reverse transcriptase, integrase, or protease enzymes, Lenacapavir exerts its antiviral activity by selectively binding to a conserved site on the HIV-1 capsid protein. This unique binding interferes with multiple critical phases of the viral replication cycle, including:
Capsid assembly and disassembly
Nuclear import of the viral genome
Genome encapsidation during replication
By targeting several stages of the viral lifecycle, Lenacapavir effectively inhibits HIV replication while reducing the likelihood of resistance development.
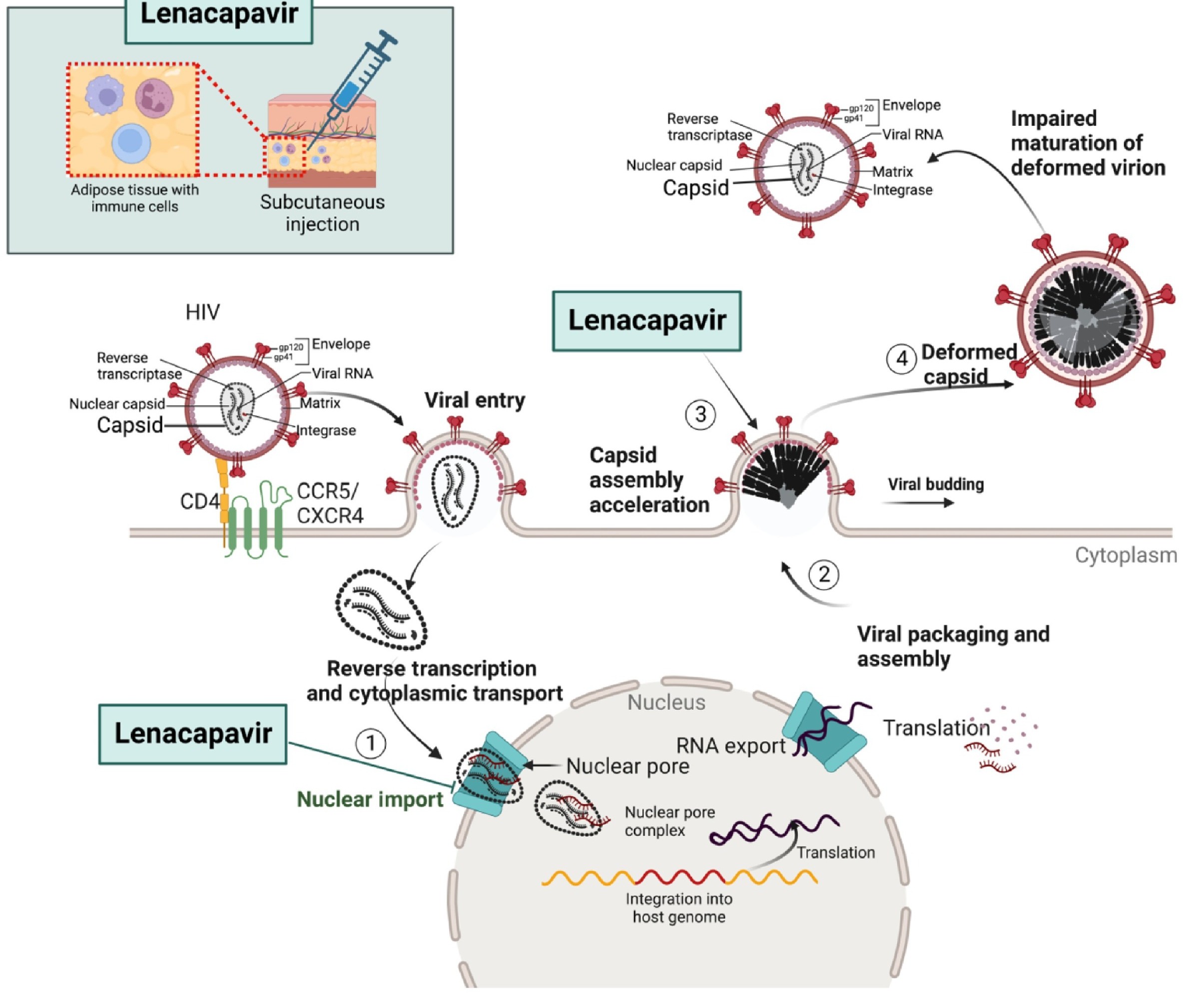
Pharmacokinetics: The Long-Acting Advantage3,4.
Lenacapavir is administered via subcutaneous injection every six months, offering an unprecedented dosing interval compared to daily oral antiretrovirals. Following an oral loading dose, the injectable formulation maintains therapeutic plasma levels for six months, sustaining drug concentrations well above the 95% inhibitory quotient (IQ) against both wild-type and drug-resistant HIV strains. This ultra-long-acting profile is especially advantageous for:
Individuals with adherence difficulties
Populations with limited access to continuous healthcare
Patients experiencing treatment fatigue from daily pill intake
Clinical Trials and Efficacy
The CAPELLA trial (NCT04150068)5
This trial evaluated Lenacapavir in patients with multidrug-resistant HIV. In the randomized cohort, 88% of participants demonstrated a ≥0.5 log₁₀ reduction in HIV RNA following 14 days of Lenacapavir monotherapy. At week 26, 81% of patients achieved viral suppression (HIV-1 RNA <50 copies/mL) when Lenacapavir was administered alongside an optimized background regimen (OBR)
Purpose 1 and Purpose 2 (Phase III Trials)6
These ongoing studies are assessing Lenacapavir’s role in HIV prevention among high-risk populations, including adolescent girls, young women, men who have sex with men (MSM), and gender-diverse individuals
Key outcomes:
≥99.9% efficacy was observed—zero infections in cisgender women in Purpose 1 and only two infections among 2,179 participants in Purpose 2.
Subgroup analysis reported 96% efficacy in cisgender women and 100% in MSM and gender-diverse individuals.
Safety and Tolerability5
Lenacapavir demonstrates a favorable safety profile. Commonly reported adverse events include:
Localized injection site reactions (e.g., erythema, pain, swelling)
Mild gastrointestinal symptoms such as nausea and diarrhea
Rare occurrences of transiently elevated serum creatinine
Notably, no serious drug-related adverse events or hypersensitivity reactions were observed in clinical trials
Regulatory Status and Global Access1,7,8
FDA approval (Sunlenca®) for treatment: December 2022
EMA approval: August 2022 WHO prequalification: Currently pending
FDA approval for PrEP (Yeztugo): June 18, 2025
The FDA’s 2025 approval of Lenacapavir for twice-yearly PrEP represents a historic milestone in HIV prevention. The World Health Organization (WHO) has recognized Yeztugo’s potential as a transformative addition to the global HIV prevention arsenal
Global Access and Affordability Challenges9,10
Despite its clinical promise, accessibility remains a concern. Lenacapavir carries a U.S. annual list price of $28,218, potentially limiting its use in low-resource settings. In response, Gilead has pledged to manufacture 10 million doses by 2026 and has licensed generic production across 120 low- and middle-income countries (LMICs). However, global health advocates, including UNAIDS, continue to urge Gilead to reduce the price to an estimated $25–$40 per person-year to ensure widespread impact
Public Health Implications and Adherence6
The availability of a twice-yearly injectable PrEP addresses several key barriers in current HIV prevention strategies:
Eliminates the burden of daily oral dosing and associated stigma
Improves adherence, which remains a challenge with over 50% of daily PrEP users discontinuing within one year
Reduces the frequency of healthcare visits required for monitoring and resupply
Experts regard Lenacapavir’s discreet and long-acting profile as a potential game-changer in the effort to reduce global HIV transmission rates
Future Perspectives11
Regulatory expansion: Applications are in progress for approval in EMA, Canada, Australia, South Africa, Brazil, Argentina, and other countries
WHO guidelines: Updated guidance is expected by July 14, 2025, coinciding with the International AIDS Conference.
Next-generation development: Investigations are ongoing into annual dosing regimens and self-injectable formulations, which could further streamline HIV prevention strategies.
Conclusion
With its novel mechanism, exceptional pharmacokinetics, and demonstrated efficacy in both treatment and prevention, Lenacapavir is poised to reshape the trajectory of HIV care. However, its true public health impact will depend on equitable access, sustainable pricing models, and broad international deployment—particularly in regions with the highest burden of disease.