Acute Respiratory Distress Syndrome (ARDS)
When the Lungs Turn to Stone
It begins as a whisper — a fever, a cough, a shortness of breath. But within days, a patient who seemed healthy is gasping for air, their lungs heavy and stiff as wet cement. For clinicians, this scene is both familiar and haunting: the face of Acute Respiratory Distress Syndrome (ARDS) — one of medicine’s most feared and complex syndromes. First described in 1967 by Drs. David Ashbaugh and Tom Petty, ARDS has since become a defining challenge of critical care.1
It is a disease not of one cause, but of many: pneumonia, sepsis, trauma, aspiration, or viral infection can all ignite the same catastrophic cascade of:2
Diffuse alveolar damage
Endothelial leak
Refractory hypoxemia.
Over half a century later, our understanding has deepened. We’ve learned how to protect the lung, when to dry it, how to position it, and most recently — how to listen to its molecular voice.
The story of ARDS is the story of critical care itself: a journey from survival to precision.
Definition
The first step toward understanding any disease is naming it. In 2012, the Berlin Definition gave ARDS its modern clinical shape — classifying it by oxygenation thresholds (PaO₂/FiO₂ ratio) and standardizing research worldwide. Yet the Berlin criteria assumed access to arterial blood gases, ventilators, and CT imaging — tools abundant in Berlin or Boston, but not in Bamako or Bandung.3
The Berlin Definition of Acute Respiratory Distress Syndrome
| Parameter | Berlin Definition (2012) |
|---|---|
| Timing | Within 1 week of a known clinical insult or new/worsening respiratory symptoms |
| Chest Imaging | Bilateral opacities—not fully explained by effusions, lobar/lung collapse, or nodules |
| Origin of Edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Requires objective assessment (e.g., echocardiography) to exclude hydrostatic edema if no risk factor present. |
| Oxygenation (Severity) |
|
Source: Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307(23):2526–2533.
The COVID-19 pandemic exposed those limits. Across ICUs from Milan to Melbourne, patients on high-flow nasal oxygen (HFNO) or non-invasive ventilation (NIV) displayed every hallmark of ARDS — bilateral infiltrates, profound hypoxemia, diffuse alveolar injury — yet were excluded from diagnosis because they weren’t intubated. Thousands of “invisible” cases went uncounted, untreated by the evidence-based strategies proven to save lives.
The Global Redefinition
In response, a global task force led by Michael Matthay (UCSF), Laurent Brochard (Toronto), and Yaseen Arabi (Riyadh) reimagined ARDS for a world where technology should never limit recognition. The new global definition of ARDS preserves the core physiology but expands inclusivity:4
The Global Definition of ARDS (2024)
| Parameter | Global Definition (2024) | Key Innovation |
|---|---|---|
| Timing | ≤ 1 week of symptom onset | Same as Berlin |
| Imaging | Bilateral or unilateral opacities (X-ray, CT, or ultrasound) | Accessible diagnostics |
| Origin | Not explained by heart failure | Core principle retained |
| Oxygenation | PaO₂/FiO₂ ≤ 300 or SpO₂/FiO₂ ≤ 315 | Non-invasive equivalence |
| Support | Includes HFNO ≥ 30 L/min or NIV | Reflects real-world therapy |
| Setting | Any healthcare environment | Promotes global applicability |
This shift makes ARDS a biological, not technological diagnosis — a definition that works as well in Nairobi as in New York.
Why It Matters
By embracing lung ultrasound and the SpO₂/FiO₂ ratio, the new framework empowers clinicians even in resource-limited hospitals to diagnose and treat ARDS. It builds on the Kigali Definition5, which validated ultrasound-based detection of alveolar edema and pleural irregularities.6
As Professor Matthay summarized, “ARDS should not depend on what machine you have — only on the biology you recognize.”2
This democratization of diagnosis marks a global triumph: ARDS is no longer confined to ICUs with ventilators, but a syndrome that can be identified and managed anywhere.
Current Evidence-Based Practice
From Oxygen Toxicity to Lung Protection
The early decades of ARDS management were defined by urgency and brute force. Clinicians, desperate to oxygenate failing lungs, turned to ever-higher pressures and tidal volumes. It seemed logical — if oxygen is life, then more must be better. But over time, evidence revealed a devastating paradox: the very ventilator designed to save the lung could, in fact, destroy it.
That realization began in the 1970s at the University of California, San Francisco, where Webb and Tierney conducted a now-classic series of experiments. They found that high airway pressures without positive end-expiratory pressure (PEEP) caused the fragile alveoli to rupture, while the simple addition of modest PEEP prevented that collapse. The insight was transformative — oxygen itself was not the enemy; overdistension was. For the first time, the focus shifted from delivering air into the lungs to protecting the lungs from the air we deliver.7
ARDS Network Trial
That principle matured into clinical proof three decades later. In 2000, the landmark ARDS Network Trial changed critical care forever. The study demonstrated that patients ventilated with lower tidal volumes (6 mL/kg predicted body weight) and plateau pressures below 30 cm H₂O experienced a 22% reduction in mortality — roughly one life saved for every eleven patients treated.8
Subsequent biomarker studies revealed that gentle ventilation dampened systemic inflammation, reducing cytokines like IL-6, IL-8, and TNF-α, and lowering epithelial injury markers such as surfactant protein-D and RAGE. The ventilator, once a blunt instrument of survival, had become a precision tool of molecular healing.9,10
Surfactant Protein-D (SP-D)
What it is:
A collectin family protein produced mainly by type II alveolar epithelial cells in the lungs. It plays a key role in:
innate immunity
pathogen recognition
modulation of inflammatory responses
maintaining alveolar homeostasis
Why it matters in ARDS:
SP-D is released when alveolar epithelial cells are injured, so elevated blood levels reflect epithelial damage and worsening ARDS severity.
Receptor for Advanced Glycation End-Products (RAGE)
What it is:
A multi-ligand cell-surface receptor highly expressed on alveolar type I epithelial cells.
It participates in:
inflammation
cell signaling
oxidative stress pathways
Why it matters in ARDS:
RAGE levels rise when type I alveolar epithelium is damaged.
Thus, soluble RAGE in plasma is a powerful biomarker of epithelial injury and correlates with:
severity of lung injury
impaired alveolar fluid clearance
worse clinical outcomes
The ARDS Bundle (2025)
Two decades later, ARDS management has matured into a symphony of precision, balance, and restraint. The modern ARDS bundle integrates physiology with evidence, pairing mechanical ventilation strategies with supportive measures that optimize oxygenation and minimize harm. At its heart, modern ARDS therapy is about balance — between oxygenation and injury, pressure and protection, intervention and patience.8,11–16
Evidence-Based Therapeutic Elements in ARDS Management
| Therapeutic Element | Landmark Evidence | Effect |
|---|---|---|
| Low tidal volume (6 mL/kg PBW) | NEJM 2000 | ↓ Mortality |
| Plateau pressure < 30 cm H₂O | NEJM 2000 | ↓ Barotrauma |
| Moderate–high PEEP | JAMA 2020 meta-analysis | Prevents collapse |
| Driving pressure ≤ 15 cm H₂O | Amato et al., NEJM 2015 | Predicts survival |
| Prone positioning ≥ 16 h/day | PROSEVA, NEJM 2013 | ↓ Mortality |
| Conservative fluid strategy | FACTT, NEJM 2006 | ↓ Edema |
| Balanced crystalloids (e.g., Plasmalyte) | SMART, NEJM 2018 | Avoids acidosis |
| High-flow nasal oxygen | FLORALI, NEJM 2015 | ↓ Intubation |
| Awake proning | JAMA Intern Med 2025 | ↓ Mortality |
Among all these strategies, one remains a quiet hero: prone positioning.By turning the patient onto their abdomen, ventilation is redistributed, perfusion improves, and dorsal lung units that once collapsed now re-engage in gas exchange. It is a simple act, grounded in elegant physiology, and yet it continues to save lives around the world — a reminder that even in an age of molecular precision, sometimes the most powerful medicine is gravity itself.17
Still, even with meticulous adherence to evidence-based practice, one unsettling truth persists: two patients with the same clinical profile can respond to treatment in dramatically different ways.This inconsistency — long dismissed as clinical variability — may, in fact, be biology speaking in different dialects. To truly understand ARDS, the field had to look beyond the ventilator and into the molecular fabric of the disease itself.
The Molecular Faces of ARDS
One Syndrome, Two Biologies
It began with a simple but profound observation: not all ARDS is the same. Two patients might arrive with identical oxygen deficits, radiographic infiltrates, and ventilator settings, yet their bodies behave as if fighting two entirely different battles. Through latent class analysis of over 12,000 patients, researchers at UCSF and the NIH uncovered a striking dichotomy — two reproducible biological phenotypes below that reshaped everything we thought we knew about this syndrome.18–21
- Hyperinflammatory ARDS — marked by elevated IL-6, IL-8, TNFR-1, and PAI-1, profound endothelial leak, mitochondrial dysfunction, and mortality rates reaching 45–70%.
What is PAI-1?
[Plasminogen Activator Inhibitor-1 (PAI-1)] is a serine protease inhibitor (SERPIN) that regulates the body’s fibrinolytic system, which is responsible for breaking down blood clots.
Its main job:
PAI-1 inhibits two key enzymes:
tPA (tissue plasminogen activator)
uPA (urokinase plasminogen activator)
These enzymes normally convert plasminogen → plasmin, the enzyme that dissolves fibrin clots.
So when PAI-1 is high, fibrinolysis decreases, leading to:
fibrin deposition
microvascular thrombosis
impaired oxygenation
endothelial dysfunction
- Hypoinflammatory ARDS — characterized by lower cytokine activity, preserved mitochondrial function, and mortality closer to 18–30%.
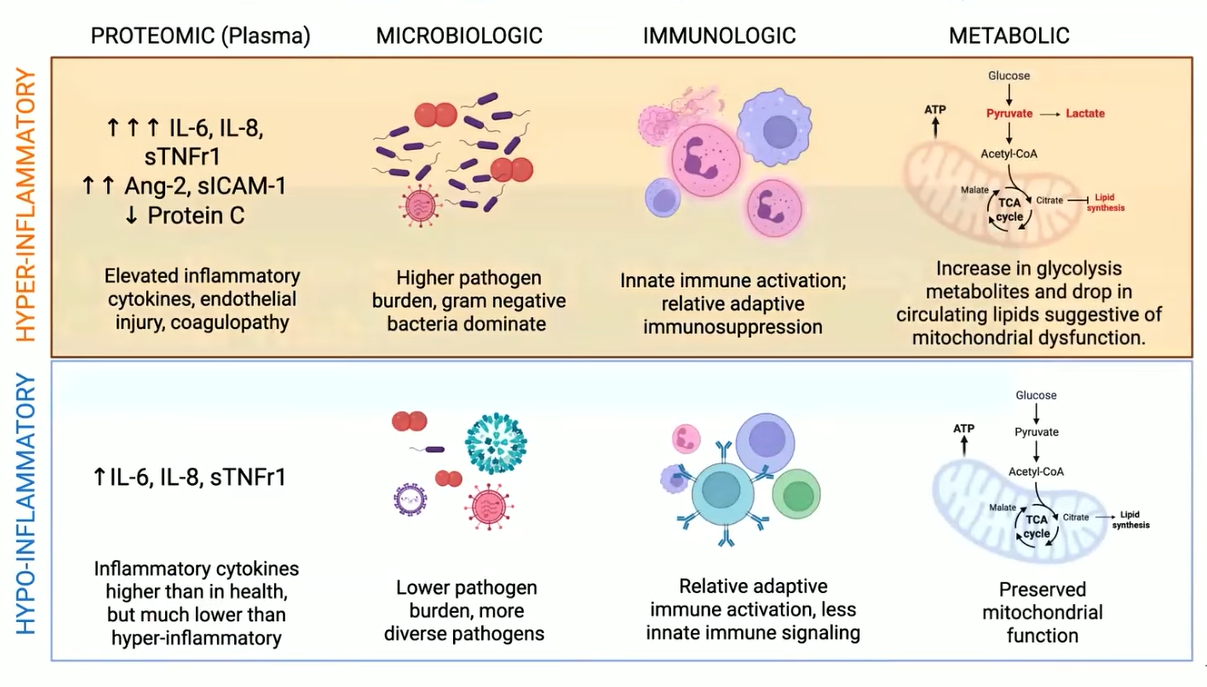
Pathogenesis:of Inflammatory Phenotypes.
This revelation reframed decades of so-called negative clinical trials. Many therapies once dismissed as ineffective — from statins to corticosteroids to high PEEP strategies — may simply have failed because they were tested in mixed populations, where opposite biologies cancelled each other out. What seemed like inconsistency was, in truth, a signal blurred by heterogeneity.22–24
The story doesn’t end in the alveoli. The same inflammatory phenotypes appear across other forms of critical illness — most notably in sepsis — suggesting that ARDS is not purely a lung disease, but a systemic immune expression of organ failure.25
The distinction mirrors that of Th2-high and Th2-low asthma, where immune signatures dictate response to therapy.26,27 Under the microscope, the body’s response to injury is not uniform but patterned — two immune logics playing out under a single clinical label. Recognizing those logics brings critical care closer to the level of precision long achieved in oncology and immunology.
Omics: Reading the Lung’s Molecular Diary
If physiology tells us how ARDS behaves, molecular science is beginning to tell us why. Advances in multi-omics technologies — genomics, transcriptomics, proteomics, metabolomics, and metagenomics — are revealing the disease’s inner choreography with unprecedented detail.28
Metagenomics captures both pathogen and host response, helping distinguish infection-driven from sterile inflammation.29
Proteomics uncovers cytokine cascades and vascular injury mediators such as angiopoietin-2 and protein C, providing a biochemical fingerprint of endothelial distress.30,31
Metabolomics identifies the glycolytic “energy collapse” that marks the hyperinflammatory phenotype.32
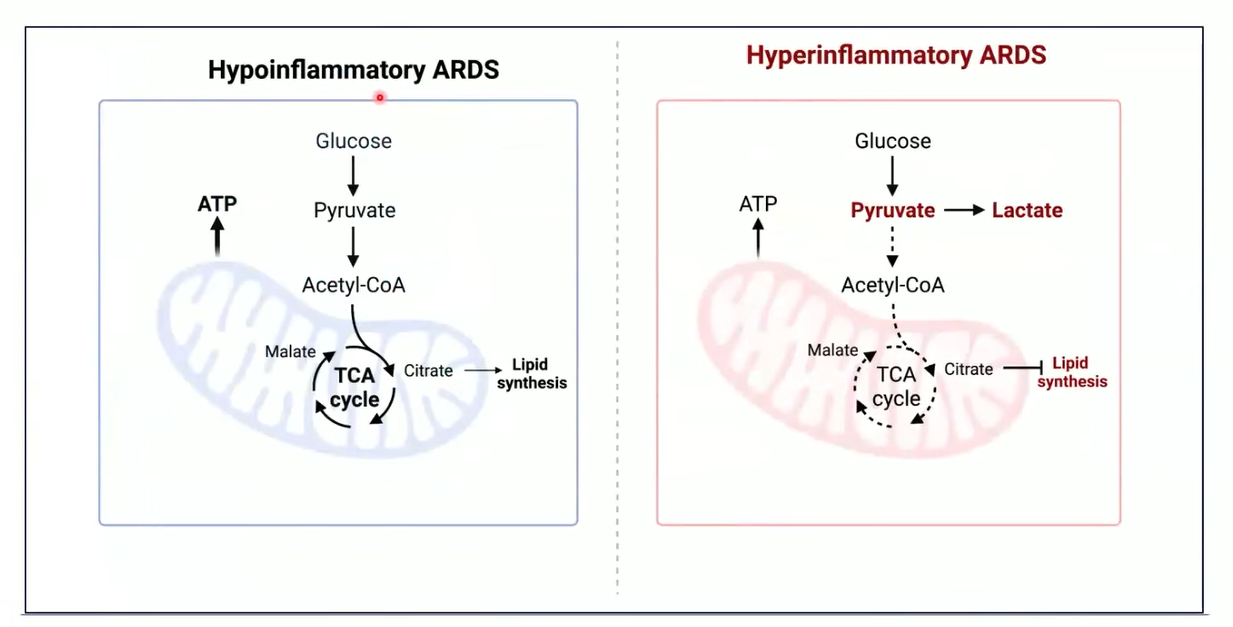
Hyperinflammation phenotype:experiences a glycolytic shift associated with mitochondrial dysfuction
Transcriptomics maps the immune landscape — distinguishing immune exhaustion from activation, revealing when inflammation becomes self-perpetuating.33
Each layer adds depth to what was once a two-dimensional diagnosis. Together, these discoveries are transforming ARDS from a physiologic syndrome into a molecular narrative — a story written in cytokines, metabolites, and gene signatures. And within that story lies the potential to guide therapy not just by symptoms or numbers, but by the biology of each individual patient.
The Future of ARDS
The Era of Precision Critical Care
Critical care medicine stands at a turning point and we are entering the third era of critical care — the era of precision. In the early days, clinicians treated syndromes; now, we are beginning to treat mechanisms. The intensive care unit is no longer just a ward for survival — it is becoming a living laboratory, where molecular biology, data science, and bedside medicine converge to dictate individualized therapy.34,35
From Discovery to Implementation
This transformation began with an uncomfortable truth uncovered by retrospective studies: ARDS is not one disease but many, united only by a final common pathway of lung injury. Patients with identical chest X-rays can have profoundly different inflammatory profiles — and respond to therapies in opposite ways. Recognizing this heterogeneity was the first breakthrough; the next step is proving that treating these biological differences can change outcomes. Yet the path from discovery to implementation has not been straightforward.36,37
Clinicians now face a practical challenge:
How can we classify ARDS subtypes in real time, at the bedside, when lives depend on minutes rather than days of lab processing?
Rapid Phenotyping and the PHIND Study
Here, machine learning became the bridge between molecular complexity and clinical practicality. In 2020, Sinha and colleagues demonstrated that a parsimonious three-variable model — interleukin-8, bicarbonate, and protein C — could predict ARDS phenotypes with nearly 90% accuracy. This was more than an algorithm; it was a proof of concept that precision could be simple.38
By 2025, this concept was tested on a global scale in the PHIND trial, an observational cohort spanning 20 hospitals and over 1,800 patients. The results were striking: clinicians achieved real-time phenotyping in more than 95% of cases using bedside assays, and mortality differences faithfully reproduced those seen in earlier biomarker studies — 51% in hyperinflammatory vs. 28% in hypoinflammatory ARDS.39
The message was clear: precision medicine is not just a research ideal — it is now clinically feasible.
PANTHER trial
Building on that success, the field is now setting its sights even higher. The PANTHER trial (Precision Medicine Adaptive Network Platform in Hypoxemic Respiratory Failure) represents a new generation of global, Bayesian adaptive platform trials. By enrolling patients according to biological phenotype, PANTHER will test targeted therapies — beginning with simvastatin and baricitinib — against standard care. Unlike conventional studies, adaptive platforms evolve in real time, dropping ineffective arms and expanding promising ones.
Enrollment begins in late 2025 across the UK, with expansion to North America, Europe, Japan, and Australia. If successful, PANTHER may deliver the first definitive proof that biology-guided therapy can improve survival in critical illness — a moonshot moment for intensive care medicine.
No Single “Best” Phenotype — Only Treatable Traits
As the science deepens, humility must keep pace with progress. Researchers increasingly acknowledge that there may never be a single “best” way to classify ARDS. Each patient, shaped by their unique biology, may represent their own phenotype. What truly matters is not labeling, but identifying traits that predict response to therapy — what precision medicine calls treatable traits. This philosophy, borrowed from oncology and asthma, is now taking root in critical care: the idea that the future lies not in defining rigid categories, but in matching therapies to biological behavior.40,41
Together, these advances signal a profound shift. The ICU of the past fought for survival; the ICU of the future will fight for specificity — using molecular insight as its compass. In this new era of precision critical care, medicine moves closer than ever to its ideal: the right treatment, for the right patient, at the right moment.
Conclusion
ARDS began as a clinical puzzle and has evolved into a global scientific effort that brings together physiology, technology, and molecular biology. Ventilation strategies are now gentler, diagnostic criteria more inclusive, and our understanding of the immune and inflammatory landscape far more refined. What was once a purely clinical syndrome is now recognized as a set of distinct biological states, each with its own pathways and treatment implications.
This shift marks a new direction in critical care. Management is increasingly guided by the molecular signatures that shape disease rather than by a single, uniform definition. As bedside practice incorporates real-time biomarkers and computational tools, ARDS is transitioning from broad supportive therapy to targeted, biologically informed care.
The future of ARDS will be defined by this integration of clinical insight and molecular precision — a movement toward treatments tailored to the biology unfolding within each patient.