
Alzheimer’s Disease and Dementia
Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disorder, representing the leading cause of dementia and accounting for approximately two-thirds of all cases. It is pathologically characterized by the accumulation of extracellular amyloid-β plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein. These pathological hallmarks contribute to widespread neuronal degeneration and synaptic loss, ultimately resulting in significant cerebral atrophy1. Notably, these neurobiological changes can begin decades prior to the onset of clinical symptoms, posing substantial challenges to timely diagnosis and therapeutic intervention. Despite ongoing advances in research, there is currently no cure for Alzheimer’s disease; thus, early detection and a comprehensive understanding of its molecular underpinnings remain critical for the development of effective disease-modifying treatments.The figure below clearly depict the normal and Alzheimer brain2.
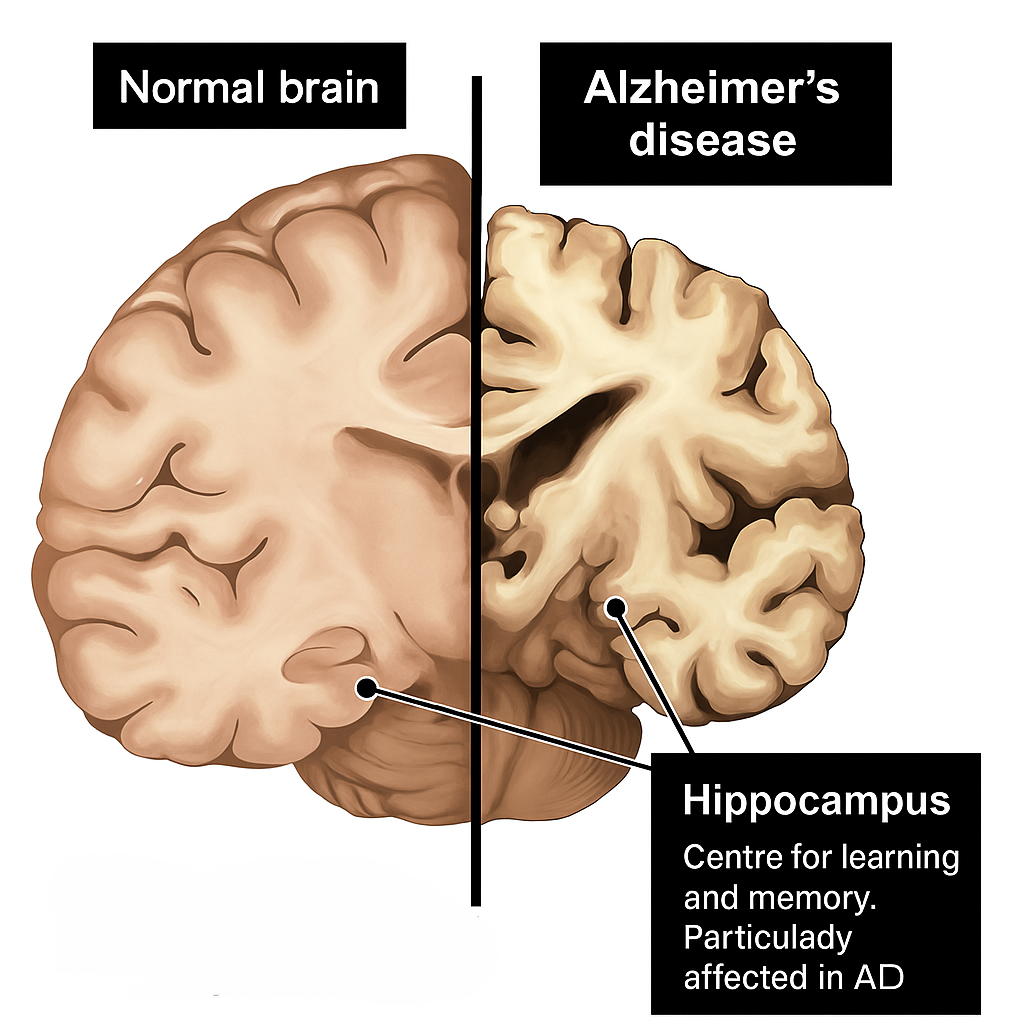
The `Hippocampus: a critical hub for memory consolidation, is one of the earliest and most severely affected regions in Alzheimer’s disease, undergoing profound atrophy that correlates directly with the progression of cognitive decline.
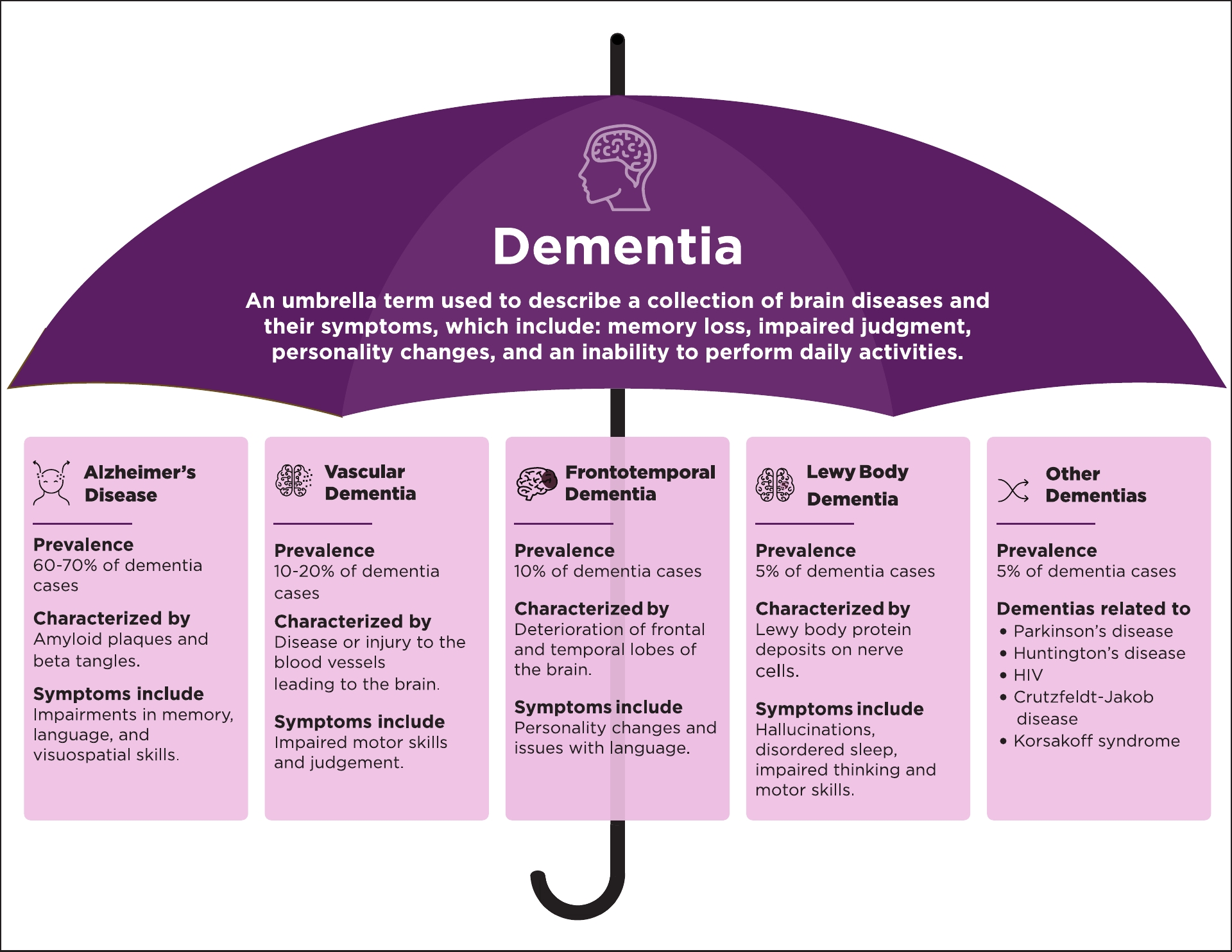
Importantly, dementia itself is not a singular disease, but a broad clinical syndrome comprising a decline in memory, executive function, language, visuospatial skills, and social cognition. This syndrome arises from a variety of pathophysiological processes, including Alzheimer’s disease, vascular dementia, frontotemporal dementia, and dementia with Lewy bodies. Each of these forms is associated with distinct neuropathological substrates, clinical features, and disease trajectories.3
Core Symptoms of Dementia
- Memory impairment
- Disorientation
- Language disturbances (aphasia)
- Impaired executive function
- Impaired judgment and reasoning
- Visuospatial deficits
- Decline in ADLs (activities of daily living)
- Personality and behavioral changes
Global Statistics and Prevalence
Alzheimer’s disease (AD) and other dementias represent one of the most urgent global health challenges of the 21st century. According to the World Health Organization (WHO), more than 55 million people worldwide were living with dementia as of 2023, with Alzheimer’s disease accounting for approximately 60–70% of all cases. The global burden is projected to increase dramatically, reaching an estimated 78 million by 2030 and 139 million by 2050, driven primarily by aging populations in both high- and low-to-middle-income countries.
The prevalence of AD increases exponentially with age: while it affects approximately 5% of individuals aged 65–74, this figure rises to 13.1% among those 75–84, and exceeds 33% in individuals over 85. Notably, the disease disproportionately impacts women, who make up nearly two-thirds of those diagnosed, possibly due to longer life expectancy and hormonal influences.
Economic costs are also substantial, with global dementia-related expenditures estimated at over USD 1.3 trillion annually—expected to surpass USD 2.8 trillion by 2030. Beyond financial costs, dementia imposes significant emotional, physical, and psychological strain on caregivers and families, often requiring comprehensive long-term care solutions.
Historical Perspective
The medical characterization of AD began in 1906 when Dr. Alois Alzheimer presented the case of Auguste Deter, a woman exhibiting progressive cognitive deterioration. Postmortem analysis revealed the presence of neuritic plaques and neurofibrillary tangles. Concurrently, Czech psychiatrist Oskar Fischer described similar findings, thus reinforcing the pathological foundation of the disease. Their seminal contributions laid the groundwork for our modern understanding of AD as a clinicopathological entity.
Alois Alzheimer (1864–1915): was a German psychiatrist and neuropathologist who first identified the neurodegenerative condition now known as Alzheimer’s disease in 1906, after observing distinctive brain pathology in a patient with memory loss and cognitive decline. He worked closely with Emil Kraepelin, who later named the disease in his honor. Alzheimer’s meticulous histological studies advanced the understanding of dementia and neuropathology. His work laid the foundation for modern neurodegenerative research.
Oskar Fischer (1876–1945): was a Czech-German neuropathologist who independently studied senile dementia and described neuritic plaques around the same time as Alzheimer. Working in Prague, he analyzed dozens of brains with what we now recognize as Alzheimer pathology. Despite making significant contributions, his work was historically overshadowed by Alzheimer’s. Fischer died under unclear circumstances during World War II, possibly in a concentration camp.

Neuropathological Hallmarks
AD is histopathologically defined by two hallmark features: extracellular deposits of amyloid-beta (Aβ) forming senile plaques, and intracellular aggregates of hyperphosphorylated Tau protein resulting in neurofibrillary tangles shown below. These lesions disrupt synaptic communication, compromise neuronal metabolism, and activate glial-mediated neuroinflammation. The hippocampus, entorhinal cortex, and neocortical regions are especially vulnerable, leading to cognitive dysfunction and brain atrophy.
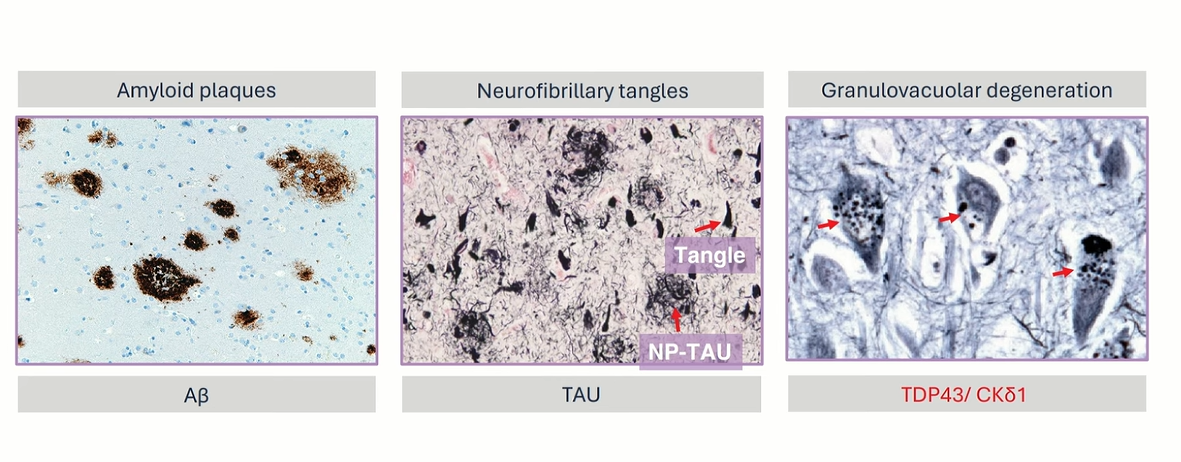
Extracelluar Plaque -Amyloid Beta ` Intracelluar Plaque -Neurofibrillary tangles
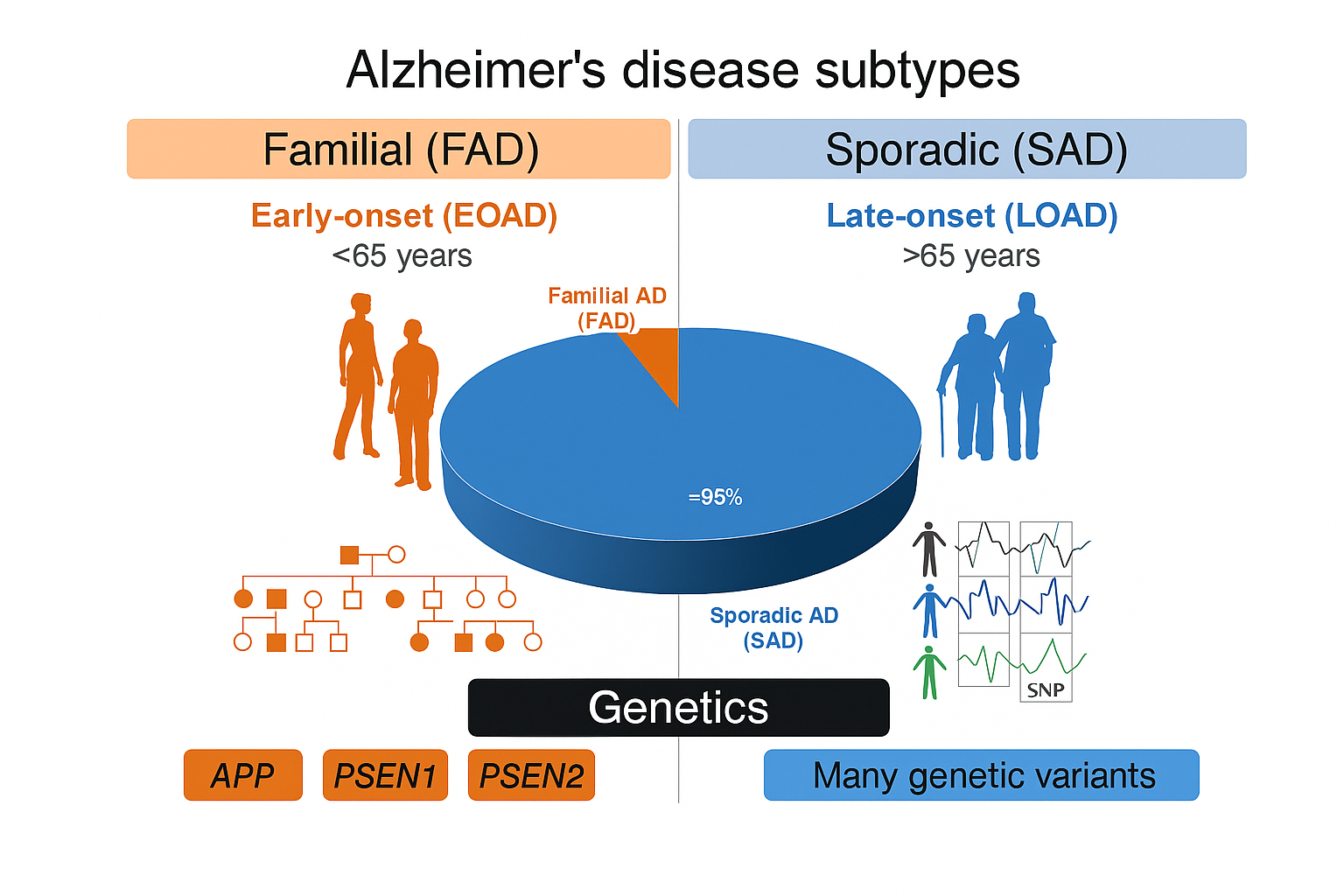
Clinical and Genetic Subtypes
AD is broadly divided into:
Familial/Early-Onset AD (EOAD): Occurring before age 65, EOAD is often inherited in an autosomal dominant pattern involving mutations in the APP, PSEN1, or PSEN2 genes.
Sporadic/Late-Onset AD (LOAD): This more common subtype manifests after age 65 and results from polygenic and environmental interactions. The APOE ε4 allele is the strongest known genetic risk factor for LOAD.
Click below to view figure – which summarizes the types and their prevalance.
Click to View
Etiological Landscape4,5
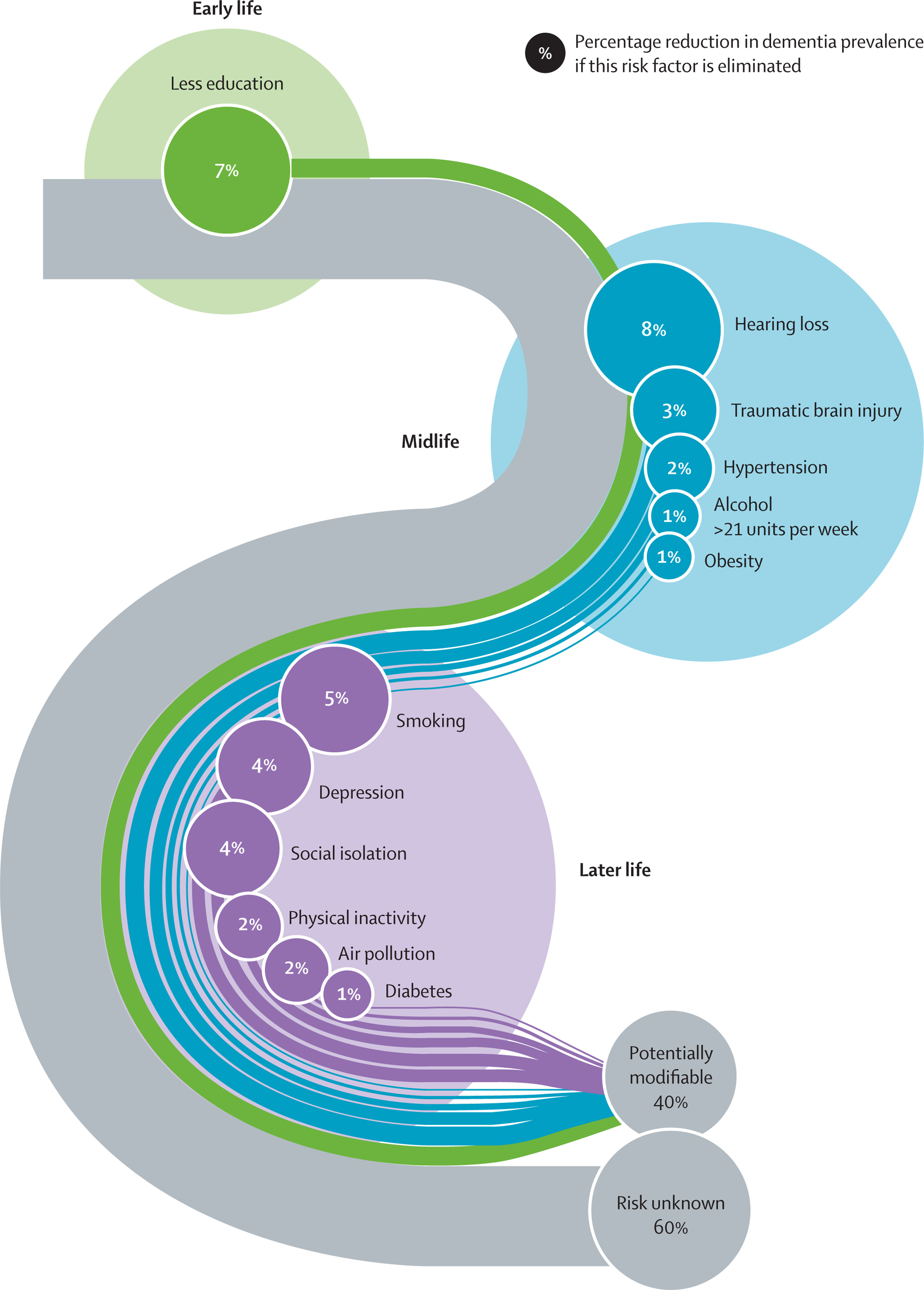
Dementia, including Alzheimer’s disease, results from a multifactorial interplay between genetic, environmental, and lifestyle influences. Increasing evidence supports that up to 40% of dementia cases may be preventable through modification of key risk factors distributed across early life, midlife, and later life. The remaining 60% of cases are attributed to unknown or non-modifiable factors, such as genetics and complex biological mechanisms yet to be elucidated.
Early Life Risk Factor
Less Education (7%)
Limited educational attainment during early developmental stages (e.g., childhood to adolescence) is associated with lower cognitive reserve—a protective mechanism whereby higher baseline cognitive function may delay clinical dementia symptoms despite underlying neuropathology.
Mechanism: Education may enhance neural plasticity, increase synaptic density, and improve compensatory capacity of the brain.
Midlife Risk Factors
Midlife (typically 45–65 years of age) is considered a critical window for intervention.
Hearing Loss (8%)
Associated with social withdrawal, depression, and accelerated brain atrophy. Early use of hearing aids or auditory rehabilitation may mitigate this risk.
Traumatic Brain Injury (TBI) (3%)
Contributes to tauopathy, chronic neuroinflammation, and increased deposition of amyloid-β plaques. Preventative strategies include helmet use, fall prevention, and occupational safety.
Hypertension (2%)
Midlife vascular risk elevates cerebral small vessel disease and ischemic injury, potentiating vascular cognitive impairment. Blood pressure control (target <130/80 mmHg) is strongly recommended.
Alcohol Consumption >21 Units/Week (1%)
Chronic alcohol use is neurotoxic and associated with hippocampal atrophy. Reducing consumption to moderate levels is essential.
Obesity (1%)
Visceral obesity contributes to systemic inflammation, insulin resistance, and cerebrovascular disease. Weight management through diet and exercise is protective.
Late Life Risk Factors
Interventions in older adulthood remain impactful but may require tailored approaches.
Smoking (5%)
Increases oxidative stress, vascular injury, and promotes neurodegeneration. Smoking cessation at any stage can reduce dementia risk.
Depression (4%)
Both a risk and prodrome of dementia. Neuroinflammation, HPA axis dysregulation, and hippocampal atrophy are implicated. Early diagnosis and treatment (psychotherapy or pharmacotherapy) are beneficial.
Social Isolation (4%)
Lack of social engagement contributes to cognitive decline and emotional distress. Interventions include community programs and structured social interaction.
Physical Inactivity (2%)
Regular aerobic and resistance exercise improve cerebral perfusion, neurogenesis, and reduce β-amyloid load.
Air Pollution (2%)
Chronic exposure to particulate matter is associated with neuroinflammation and cerebrovascular pathology. While individual control is limited, public health and policy interventions are vital.
Diabetes (1%)
Poor glycemic control accelerates vascular and neurodegenerative damage. Optimizing HbA1c and avoiding hypoglycemia are essential in older adults.
Risk Unknown (60%)
Despite robust data on modifiable factors, a majority (~60%) of dementia risk remains unexplained. This may be due to:
Genetic Susceptibility (e.g., APOE ε4)
Complex gene-environment interactions
Neuroinflammatory processes and synaptic dysfunction not yet fully characterized
Epigenetic modifications, gut-brain axis alterations, and proteostatic failure
This proportion underscores the urgent need for ongoing research into novel biomarkers, molecular mechanisms, and precision prevention strategies.
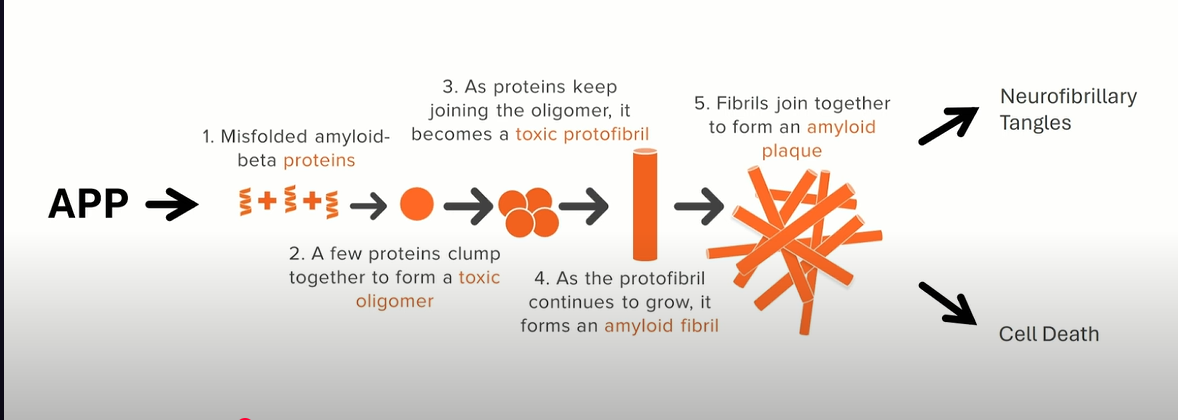
Key Hypotheses in AD Pathogenesis
Amyloid Cascade Hypothesis: Proposes that dysregulated cleavage of APP leads to Aβ42 accumulation, triggering downstream tauopathy, inflammation, and neurodegeneration.
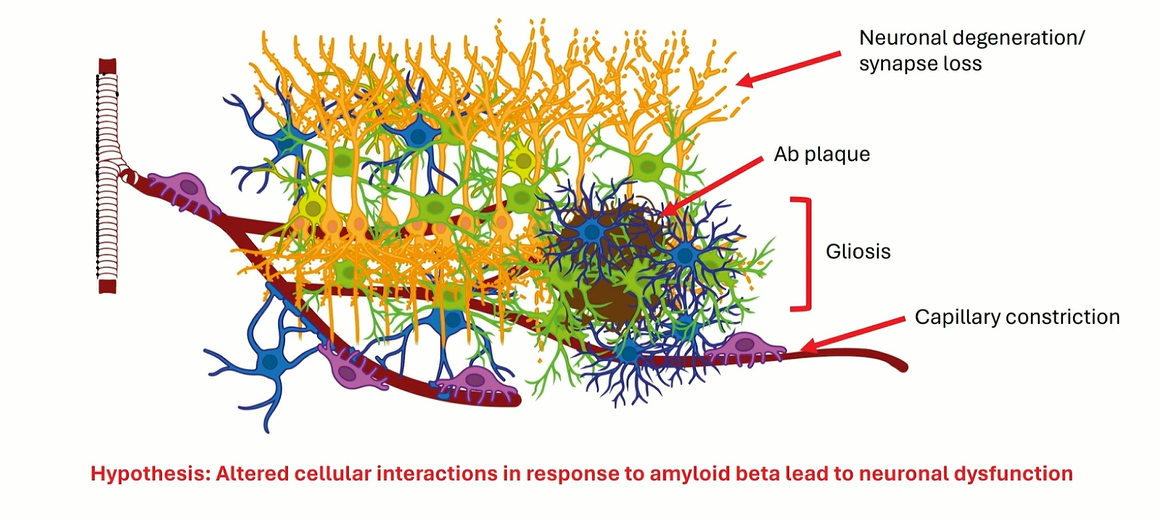
Cellular Interaction Hypothesis: Suggests that pathological glial and vascular responses exacerbate amyloid toxicity and synaptic loss.
Genetic Modulation Hypothesis: Explores how individual genetic variants influence susceptibility and progression via differential cellular responses to pathology6
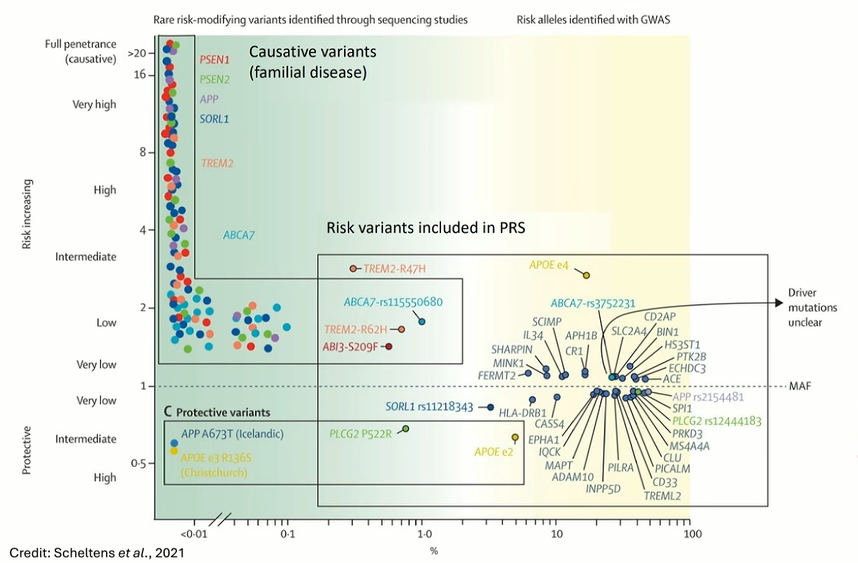
Cellular Mechanisms of AD
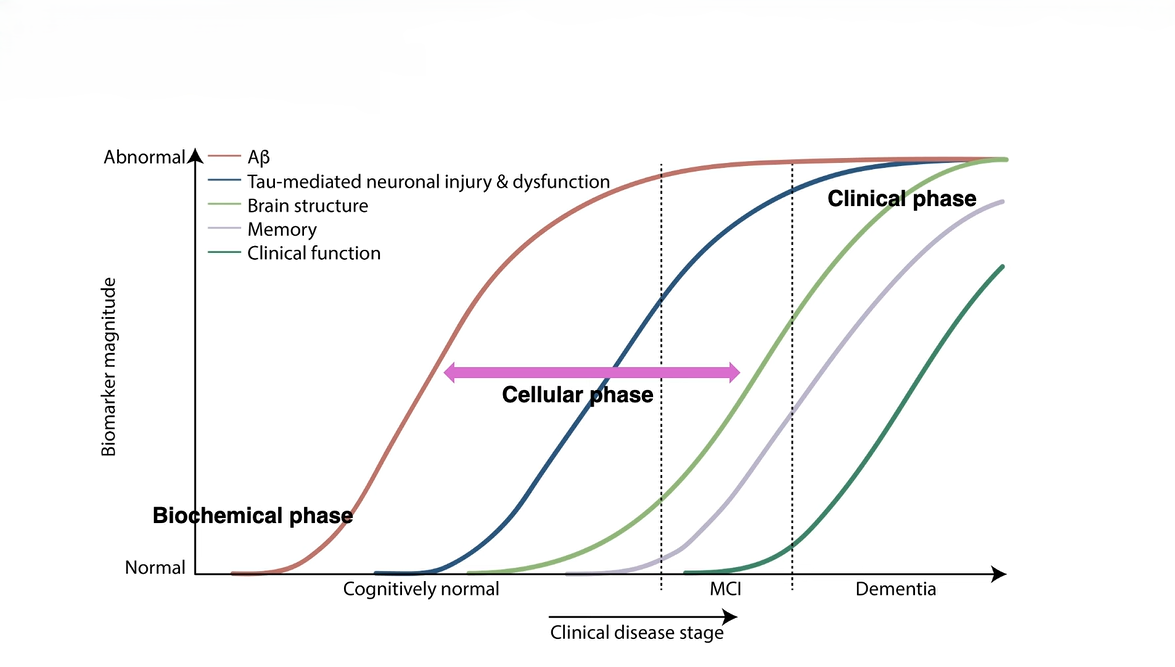
Disease Progression Phases
Biochemical Phase: Characterized by abnormal protein aggregation and mitochondrial dysregulation.
Cellular Phase: Marked by glial activation, impaired synaptic plasticity, and emerging neurodegeneration.
Clinical Phase: Cognitive decline and functional impairment dominate due to irreversible neuronal loss.
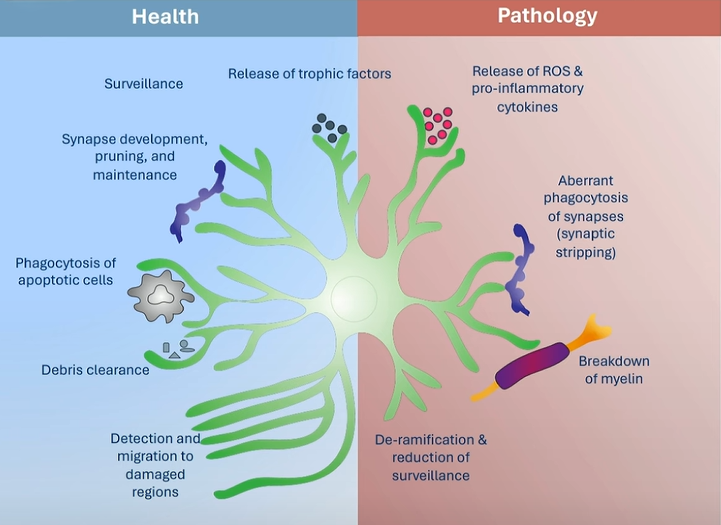
Glial Cells in AD Pathology
Microglia
In healthy brains, microglia maintain homeostasis by removing debris and regulating synaptic pruning. In AD, however, they adopt an activated phenotype (DAM: disease-associated microglia) marked by increased expression of TREM2 and ApoE. While initially protective, their chronic activation amplifies inflammation and contributes to synaptic degradation7
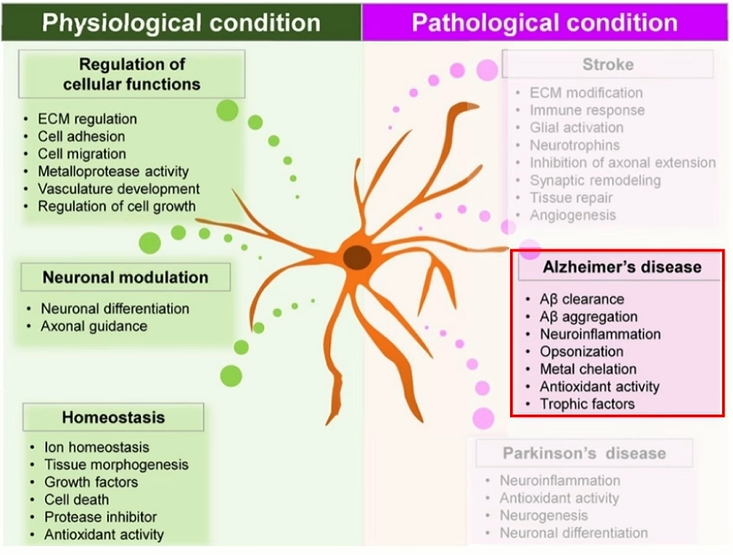
Astrocytes
Astrocytes are essential for neuronal support, neurotransmitter cycling, and blood-brain barrier maintenance. In AD, they become reactive and participate in Aβ accumulation, oxidative stress, and tau pathology propagation. Their dysfunction disrupts neuron-glial signaling and impairs metabolic coupling7.
Advances in Research
Recent technological advancements have revolutionized AD research8
Single-cell and spatial transcriptomics have elucidated cell-type-specific gene expression changes.
Proteomics and metabolomics provide insights into dynamic molecular alterations.
Multimodal imaging (e.g., amyloid and tau PET) has improved in vivo diagnosis.
Fluid biomarkerssuch as plasma p-tau217 and neurofilament light are enhancing diagnostic precision and trial design.
These innovations are redefining our understanding of disease heterogeneity, early detection, and therapeutic response.
Mitigating the Risk of AD: Non-Pharmacological Strategies
Although dementia cannot currently be cured or entirely prevented, a growing body of epidemiological and clinical evidence suggests that modifiable lifestyle and health-related factors play a significant role in reducing individual risk across the lifespan. Targeted interventions are particularly crucial in older adults, where cumulative exposures and comorbidities may amplify vulnerability to neurodegeneration.
Key Preventive Measures
Enhance Social Engagement: Regular social interaction has been shown to support cognitive reserve and delay the onset of dementia-related symptoms. Older adults should prioritize maintaining strong interpersonal networks through community activities, familial engagement, or structured social programs.
Adopt a Neuroprotective Diet: Adherence to dietary patterns such as the Mediterranean or MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diets—characterized by high intake of vegetables, fruits, whole grains, lean proteins, and healthy fats—has been associated with reduced cognitive decline and decreased dementia incidence.
Cease Tobacco Use: Smoking is an established risk factor for both vascular and neurodegenerative forms of dementia. Cessation reduces cerebrovascular burden and oxidative stress, improving long-term cognitive outcomes.
Engage in Regular Physical Activity: Sustained aerobic and resistance exercise promotes neurogenesis, enhances cerebral perfusion, and mitigates metabolic and cardiovascular risk—all of which contribute to cognitive preservation.
Optimize Management of Chronic Conditions: Proactive treatment of diabetes mellitus, hypertension, dyslipidemia, and atrial fibrillation is essential, as these conditions contribute to vascular pathology and neuroinflammation implicated in dementia etiology.
High-Risk Populations
Individuals with a positive family history of Alzheimer’s disease or other dementias, carriers of high-risk genetic alleles (e.g., APOE ε4), and those with histories of depression, substance use disorders, or traumatic brain injury are at significantly elevated risk. These populations may benefit from personalized risk assessment and earlier clinical monitoring programs.
Therapeutic Landscape9–11
Aducanumab
Mechanism:
Aducanumab is a recombinant human IgG1 monoclonal antibody that selectively binds to aggregated amyloid-beta (Aβ) plaques in the brain. It targets both insoluble fibrillar Aβ and soluble oligomers, facilitating their clearance via Fc receptor-mediated phagocytosis by activated microglia. This process is hypothesized to mitigate amyloid-driven neurotoxicity and halt further synaptic dysfunction
Dose: 10 mg/kg IV every 4 weeks.
Duration: Continuous, dependent on benefit-risk balance.
Adverse Events: The most significant adverse effect associated with aducanumab is Amyloid-Related Imaging Abnormalities (ARIA), particularly ARIA-E (vasogenic edema) and ARIA-H (microhemorrhages). Additional side effects include headache, dizziness, nausea, and confusion. ARIA is dose-dependent and more prevalent in APOE ε4 carriers.
Donanemab
Mechanism: Donanemab is a humanized IgG1 monoclonal antibody that targets a specific pyroglutamate-modified epitope of Aβ (N3pG), which is enriched in mature plaques. Its binding facilitates plaque clearance via microglial phagocytosis, leading to robust and rapid amyloid reduction as evidenced by amyloid-PET imaging
Dose: 700 mg IV x3, then 1400 mg monthly.
Duration: May stop after PET-confirmed plaque clearance.
Adverse Events: Donanemab is also associated with ARIA-E/H, particularly during the initial treatment phase. Other adverse effects include infusion-related reactions, hypersensitivity, and neuropsychiatric symptoms (e.g., agitation or hallucinations). Serious events may require dose adjustment or therapy discontinuation..
Lecanemab
Mechanism: Lecanemab is a humanized IgG1 monoclonal antibody that selectively binds soluble Aβ protofibrils, a toxic oligomeric form implicated in early synaptotoxicity. By targeting protofibrils, lecanemab aims to prevent downstream tau hyperphosphorylation, glial activation, and neuronal loss.
Dose: 10 mg/kg IV every 2 weeks.
Duration: Long-term; dependent on clinical and biomarker outcomes.
Adverse Events: Similar to other anti-Aβ therapies, ARIA-E and ARIA-H remain primary concerns, with increased incidence in APOE ε4 carriers. Infusion-related reactions, hypersensitivity reactions, and a potential increase in cardiovascular events have also been reported in clinical trials..
Pharmacists’ Role in the Management of Alzheimer’s Disease
Pharmacists play a pivotal role in the multidisciplinary care of patients with Alzheimer’s disease (AD), contributing across prevention, early detection, pharmacological management, and patient and caregiver education. Given the chronic, progressive nature of AD and the complexity of its treatment regimens, pharmacists are uniquely positioned to optimize therapeutic outcomes and enhance quality of life.
Medication Therapy Management (MTM)
Pharmacists are integral to identifying appropriate pharmacologic interventions, particularly in navigating the risks and benefits of disease-modifying therapies such as aducanumab, donanemab, and lecanemab, as well as symptomatic treatments like cholinesterase inhibitors (e.g., donepezil, rivastigmine) and NMDA receptor antagonists (e.g., memantine). They assess drug interactions, monitor for adverse events (especially Amyloid-Related Imaging Abnormalities, or ARIA), and ensure adherence to treatment protocols.
Pharmacovigilance and Safety Monitoring
Pharmacists oversee the safe administration of biologic therapies, often administered intravenously in specialized settings. Their expertise is crucial in coordinating baseline and follow-up MRI screenings to detect ARIA, interpreting lab values, and counseling patients on early signs of neurotoxicity, hypersensitivity, or cardiovascular events. They also help manage polypharmacy risks, which are prevalent among older adults with comorbidities.
Patient and Caregiver Counseling
Education is central to pharmacist responsibilities. They provide personalized guidance on:
The goals and limitations of current therapies.
The importance of consistent dosing schedules.
Recognizing side effects early and seeking timely medical attention.
Lifestyle modifications to support cognitive health (e.g., smoking cessation, physical activity, dietary adjustments).
Pharmacists also equip caregivers with strategies to manage behavioral symptoms and ensure safe medication storage and administration.
Public Health and Risk Reduction
Pharmacists promote dementia risk reduction through health education campaigns targeting modifiable risk factors such as hypertension, diabetes, smoking, and physical inactivity. In community settings, they screen for cognitive impairment using validated tools, identify patients who may benefit from further assessment, and facilitate referrals to neurologists or memory clinics.
Research and Clinical Trials
Pharmacists involved in clinical research contribute to the design and implementation of AD trials, ensuring protocol adherence, regulatory compliance, and accurate adverse event reporting. They assist in the selection of trial candidates and provide pharmacokinetic and pharmacodynamic insights into investigational therapies.
Interdisciplinary Collaboration
Pharmacists collaborate with neurologists, geriatricians, psychologists, nurses, and social workers in the development of comprehensive care plans. Their medication expertise enhances clinical decision-making and supports transitions of care across home, outpatient, and institutional settings.
Conclusion
Alzheimer’s disease (AD) represents a multifaceted and increasingly prevalent neurodegenerative disorder with profound personal, societal, and economic implications. Despite significant advances in our understanding of its molecular underpinnings—particularly the roles of amyloid-beta aggregation, tau pathology, neuroinflammation, and genetic susceptibility—the disease remains incurable. However, recent breakthroughs in diagnostic modalities, biomarker development, and the approval of disease-modifying monoclonal antibodies mark a pivotal shift toward earlier intervention and potentially slower progression.
Addressing the modifiable risk factors across the lifespan presents a compelling opportunity to reduce the global burden of dementia. Equally critical is the role of multidisciplinary care, where pharmacists serve as vital healthcare partners. Through their expertise in pharmacotherapy, patient education, safety monitoring, and public health engagement, pharmacists can significantly enhance the quality and continuity of care for individuals living with AD.
Moving forward, the integration of personalized medicine, precision diagnostics, and community-based prevention strategies will be essential to transforming the landscape of Alzheimer’s disease management. Continued investment in research, interdisciplinary collaboration, and healthcare policy reform will be paramount in addressing this complex, evolving challenge.